Metallic Ions Encapsulated in Electrospun Nanofiber for Antibacterial and Angiogenesis Function to Promote Wound Repair
- PMID: 33842486
- PMCID: PMC8027477
- DOI: 10.3389/fcell.2021.660571
Metallic Ions Encapsulated in Electrospun Nanofiber for Antibacterial and Angiogenesis Function to Promote Wound Repair
Retraction in
-
Retraction: Metallic ions encapsulated in electrospun nanofiber for antibacterial and angiogenesis function to promote wound repair.Front Cell Dev Biol. 2022 Oct 11;10:1058556. doi: 10.3389/fcell.2022.1058556. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36303607 Free PMC article.
Abstract
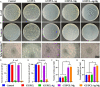
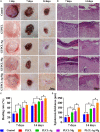
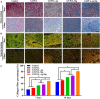
Electrospun nanofiber is an attractive biomaterial for skin tissue engineering because it mimics the natural fibrous extracellular matrix structure and creates a physical structure suitable for skin tissue regeneration. However, endowing the nanofibrous membranes with antibacterial and angiogenesis functions needs to be explored. In the current study, we aimed to fabricate gelatin/polycaprolactone (GT/PCL) (GT/PCL-Ag-Mg) nanofibers loaded with silver (Ag) and magnesium (Mg) ions for antibacterial activity and pro-angiogenesis function for wound repair. The fabricated GT/PCL membranes had a nanofibrous structure with random arrangement and achieved sustained release of Ag and Mg ions. In vitro results indicated that the GT/PCL-Ag-Mg membranes presented satisfactory cytocompatibility with cell survival and proliferation. In addition, the membranes with Ag demonstrated good antibacterial capacity to both gram-positive and gram-negative bacteria, and the Mg released from the membranes promoted the tube formation of vascular endothelial cells. Furthermore, in vivo results demonstrated that the GT/PCL-Ag-Mg membrane presented an accelerated wound healing process compared with GT/PCL membranes incorporated with either Ag or Mg ions and pure GT/PCL alone. Superior epidermis formation, vascularization, and collagen deposition were also observed in GT/PCL-Ag-Mg membrane compared with the other membranes. In conclusion, a multifunctional GT/PCL-Ag-Mg membrane was fabricated with anti-infection and pro-angiogenesis functions, serving as a potential metallic ion-based therapeutic platform for applications in wound repair.
Keywords: angiogenesis; antibacterial; electrospinning; magnesium; silver; wound healing.
Copyright © 2021 Zhu, Cao, Zhang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

