Novel Magnetic Fe3O4/α-FeOOH Nanocomposites and Their Enhanced Mechanism for Tetracycline Hydrochloride Removal in the Visible Photo-Fenton Process
- PMID: 33842779
- PMCID: PMC8028166
- DOI: 10.1021/acsomega.1c00204
Novel Magnetic Fe3O4/α-FeOOH Nanocomposites and Their Enhanced Mechanism for Tetracycline Hydrochloride Removal in the Visible Photo-Fenton Process
Abstract
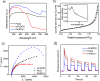
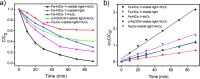
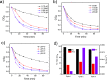
Magnetic Fe3O4/α-FeOOH heterojunction nanocomposites (denoted as Fe-NCs) have been synthesized by a fast one-pot hydrothermal method. The obtained Fe-NCs contain rich micropores with a high surface area of 135.15 m2/g. The different phases in the composites can efficiently enhance the visible-light absorption, improving the separation and transfer of photogenerated electron-hole pairs during the photocatalytic reaction. Thus, they show excellent degradation and mineralization of tetracycline (TC) over a wide pH range (5-9) in the visible photo-Fenton reaction. Especially, the catalyst exhibits the highest adsorption capacity toward TC at a neutral pH, which facilitates the surface reactions of TC with active species. Experiments evidence that the high production of photogenerated holes and superoxide radicals (O2 •-) in Fe-NCs are favorable to the high catalytic efficiency. Combined with liquid chromatography-mass spectrometry, the possible pathway toward TC degradation was proposed.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Zhao R.; Sun X.; Jin Y.; Han J.; Wang L.; Liu F. Au/Pd/g-C3N4 nanocomposites for photocatalytic degradation of tetracycline hydrochloride. J. Mater. Sci. 2019, 54, 5445–5456. 10.1007/s10853-018-03278-7. - DOI
-
- Cao J.; Xiong Z.; Lai B. Effect of initial pH on the tetracycline (TC) removal by zero-valent iron: Adsorption, oxidation and reduction. Chem. Eng. J. 2018, 343, 492–499. 10.1016/j.cej.2018.03.036. - DOI
-
- Daghrir R.; Drogui P. Tetracycline antibiotics in the environment: a review. Environ. Chem. Lett. 2013, 11, 209–227. 10.1007/s10311-013-0404-8. - DOI
-
- Lv A.; Hu C.; Nie Y.; Qu J. Catalytic ozonation of toxic pollutants over magnetic cobalt and manganese co-doped γ-Fe2O3. Appl. Catal., B 2010, 100, 62–67. 10.1016/j.apcatb.2010.07.011. - DOI
-
- Zhu J.; Shu J.; Yue X.; Su Y. Hollow and porous octacalcium phosphate superstructures mediated by the polyelectrolyte PSS: a superior removal capacity for heavy metal and antibiotics. J. Mater. Sci. 2020, 55, 7502–7517. 10.1007/s10853-020-04539-0. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

