Integrated Utilization Strategy for Soybean Oil Deodorizer Distillate: Synergically Synthesizing Biodiesel and Recovering Bioactive Compounds by a Combined Enzymatic Process and Molecular Distillation
- PMID: 33842783
- PMCID: PMC8028127
- DOI: 10.1021/acsomega.1c00333
Integrated Utilization Strategy for Soybean Oil Deodorizer Distillate: Synergically Synthesizing Biodiesel and Recovering Bioactive Compounds by a Combined Enzymatic Process and Molecular Distillation
Abstract
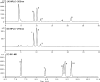
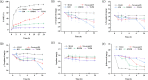
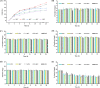
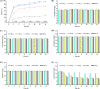
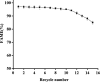
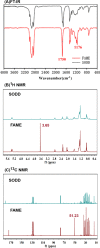
Soybean oil deodorizer distillate (SODD) is well recognized as a good source of both biodiesel and high-value bioactive compounds of tocopherols, squalene, and phytosterols. To achieve a one-step synthesis of biodiesel and recovery of bioactive compounds from SODD, four commercial immobilized enzymes (Novozym 435, Lipozyme TLIM, Lipozyme RMIM, and Lipozyme RM) and one self-prepared immobilized lipase MAS1-H108A were compared. The results showed that immobilized lipase MAS1-H108A due to the better methanol tolerance and higher catalytic activity gave the highest biodiesel yield of 97.08% under the optimized conditions: molar ratio of 1:2 (oil/methanol), temperature of 35 °C, and enzyme loading of 35 U/g SODD, even after 10 persistent cycles without significant decrease of activity. Simultaneously, there was no loss of tocopherols and squalene in SODD during the enzymatic reaction. Pure biodiesel (characterized by fourier transform infrared (FT-IR) and nuclear magnetic resonance (NMR)) and a high concentration of bioactive compounds could be successfully separated by molecular distillation at 100 °C. In a word, this work provides an interesting idea to achieve environmentally friendly treatment of SODD by combining an enzymatic process and molecular distillation, and it is suitable for industrial production.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Immobilization of Lipozyme TL 100L for methyl esterification of soybean oil deodorizer distillate.3 Biotech. 2020 Feb;10(2):51. doi: 10.1007/s13205-019-2028-6. Epub 2020 Jan 18. 3 Biotech. 2020. PMID: 32002342 Free PMC article.
-
One-step synthesis of high-yield biodiesel from waste cooking oils by a novel and highly methanol-tolerant immobilized lipase.Bioresour Technol. 2017 Jul;235:18-24. doi: 10.1016/j.biortech.2017.03.086. Epub 2017 Mar 18. Bioresour Technol. 2017. PMID: 28351728
-
Fermentation of soybean oil deodorizer distillate with Candida tropicalis to concentrate phytosterols and to produce sterols-rich yeast cells.J Ind Microbiol Biotechnol. 2014 Mar;41(3):579-84. doi: 10.1007/s10295-013-1384-1. Epub 2013 Dec 3. J Ind Microbiol Biotechnol. 2014. PMID: 24297326
-
Biodiesel fuel production by the transesterification reaction of soybean oil using immobilized lipase.Appl Biochem Biotechnol. 2007 Apr;137-140(1-12):105-14. doi: 10.1007/s12010-007-9043-5. Appl Biochem Biotechnol. 2007. PMID: 18478380
-
Vegetable Oil Deodorizer Distillate: A Rich Source of the Natural Bioactive Components.J Oleo Sci. 2016 Dec 1;65(12):957-966. doi: 10.5650/jos.ess16125. Epub 2016 Nov 9. J Oleo Sci. 2016. PMID: 27829614 Review.
Cited by
-
Annexin A7 and Its Related Protein Suppressor of Death Domains Regulates Migration and Proliferation of Hca-P Cells.Cell J. 2023 Nov 1;25(11):801-808. doi: 10.22074/cellj.2023.559724.1108. Cell J. 2023. PMID: 38071412 Free PMC article.
-
Study of vegetable oils and their blends using infrared reflectance spectroscopy and refractometry.Food Chem X. 2022 Jul 8;17:100386. doi: 10.1016/j.fochx.2022.100386. eCollection 2023 Mar 30. Food Chem X. 2022. PMID: 36974180 Free PMC article.
-
Use of hydroxyapatite as a support in the immobilization of Thermomyces lanuginosus lipase for application in the production of biodiesel using a by-product as lipid raw material.3 Biotech. 2024 Jun;14(6):163. doi: 10.1007/s13205-024-04008-4. Epub 2024 May 26. 3 Biotech. 2024. PMID: 38808300 Free PMC article.
References
-
- Yin X.; Duan X.; You Q.; Dai C.; Tan Z.; Zhu X. Biodiesel production from soybean oil deodorizer distillate usingcalcined duck eggshell as catalyst. Energy Convers. Manage. 2016, 112, 199–207. 10.1016/j.enconman.2016.01.026. - DOI
-
- Abdul Kapor N. Z.; Maniam G. P.; Rahim M. H. A.; Yusoff M. M. Palm fatty acid distillate as a potential source for biodiesel production-a review. J. Cleaner Prod. 2017, 143, 1–9. 10.1016/j.jclepro.2016.12.163. - DOI
-
- Visioli L. J.; de Castilhos F.; Cardozo-Filho L.; de Mello B. T. F.; da Silva C. Production of esters from soybean oil deodorizer distillate in pressurized ethanol. Fuel Process. Technol. 2016, 149, 326–331. 10.1016/j.fuproc.2016.04.038. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

