Detection of β-amyloid positivity in Alzheimer's Disease Neuroimaging Initiative participants with demographics, cognition, MRI and plasma biomarkers
- PMID: 33842885
- PMCID: PMC8023542
- DOI: 10.1093/braincomms/fcab008
Detection of β-amyloid positivity in Alzheimer's Disease Neuroimaging Initiative participants with demographics, cognition, MRI and plasma biomarkers
Abstract
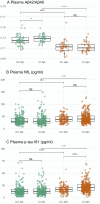
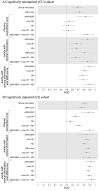
In vivo gold standard for the ante-mortem assessment of brain β-amyloid pathology is currently β-amyloid positron emission tomography or cerebrospinal fluid measures of β-amyloid42 or the β-amyloid42/β-amyloid40 ratio. The widespread acceptance of a biomarker classification scheme for the Alzheimer's disease continuum has ignited interest in more affordable and accessible approaches to detect Alzheimer's disease β-amyloid pathology, a process that often slows down the recruitment into, and adds to the cost of, clinical trials. Recently, there has been considerable excitement concerning the value of blood biomarkers. Leveraging multidisciplinary data from cognitively unimpaired participants and participants with mild cognitive impairment recruited by the multisite biomarker study of Alzheimer's Disease Neuroimaging Initiative, here we assessed to what extent plasma β-amyloid42/β-amyloid40, neurofilament light and phosphorylated-tau at threonine-181 biomarkers detect the presence of β-amyloid pathology, and to what extent the addition of clinical information such as demographic data, APOE genotype, cognitive assessments and MRI can assist plasma biomarkers in detecting β-amyloid-positivity. Our results confirm plasma β-amyloid42/β-amyloid40 as a robust biomarker of brain β-amyloid-positivity (area under curve, 0.80-0.87). Plasma phosphorylated-tau at threonine-181 detected β-amyloid-positivity only in the cognitively impaired with a moderate area under curve of 0.67, whereas plasma neurofilament light did not detect β-amyloid-positivity in either group of participants. Clinical information as well as MRI-score independently detected positron emission tomography β-amyloid-positivity in both cognitively unimpaired and impaired (area under curve, 0.69-0.81). Clinical information, particularly APOE ε4 status, enhanced the performance of plasma biomarkers in the detection of positron emission tomography β-amyloid-positivity by 0.06-0.14 units of area under curve for cognitively unimpaired, and by 0.21-0.25 units for cognitively impaired; and further enhancement of these models with an MRI-score of β-amyloid-positivity yielded an additional improvement of 0.04-0.11 units of area under curve for cognitively unimpaired and 0.05-0.09 units for cognitively impaired. Taken together, these multi-disciplinary results suggest that when combined with clinical information, plasma phosphorylated-tau at threonine-181 and neurofilament light biomarkers, and an MRI-score could effectively identify β-amyloid+ cognitively unimpaired and impaired (area under curve, 0.80-0.90). Yet, when the MRI-score is considered in combination with clinical information, plasma phosphorylated-tau at threonine-181 and plasma neurofilament light have minimal added value for detecting β-amyloid-positivity. Our systematic comparison of β-amyloid-positivity detection models identified effective combinations of demographics, APOE, global cognition, MRI and plasma biomarkers. Promising minimally invasive and low-cost predictors such as plasma biomarkers of β-amyloid42/β-amyloid40 may be improved by age and APOE genotype.
Keywords: Alzheimer’s; MRI; PET; plasma; β-amyloid.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Aisen PS. Editorial: failure after failure. What next in AD drug development? J Prev Alzheimers Dis 2019; 6: 150. - PubMed
-
- Altomare D de Wilde A Ossenkoppele R Pelkmans W Bouwman F Groot C, et al. Applying the ATN scheme in a memory clinic population. The ABIDE Project 2019; 93: e1635–46. - PubMed
-
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 2020; 16: 391–460. - PubMed
-
- Ansart M Epelbaum S Gagliardi G Colliot O Dormont D Dubois B, for the Alzheimer’s Disease Neuroimaging Initiative and the INSIGHT-preAD study, et al. Reduction of recruitment costs in preclinical AD trials: validation of automatic pre-screening algorithm for brain amyloidosis. Stat Methods Med Res 2020; 29: 151–64. - PubMed
-
- Ashford MT Veitch DP, Neuhaus J, Nosheny RL, Tosun D Weiner MW. The search for a convenient procedure to detect one of the earliest signs of Alzheimer’s disease: a systematic review of the prediction of brain amyloid status. Alzheimers Dement 2021; 1–22. doi: 10.1002/alz.12253. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
