The Evolving Immunotherapy Landscape and the Epidemiology, Diagnosis, and Management of Cardiotoxicity: JACC: CardioOncology Primer
- PMID: 33842895
- PMCID: PMC8034586
- DOI: 10.1016/j.jaccao.2020.11.012
The Evolving Immunotherapy Landscape and the Epidemiology, Diagnosis, and Management of Cardiotoxicity: JACC: CardioOncology Primer
Abstract
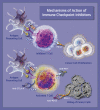
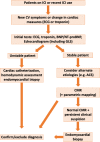
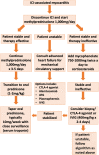
Immune checkpoint inhibitors (ICIs) are newer therapies being applied to an increasing number of patients with cancer. Data suggest that up to 36% of cancer patients may be eligible for immunotherapy and, in late 2019, there were more than 3,362 clinical trials initiated to evaluate the effectiveness of immunotherapy, either as single agents or in combination with other immunotherapy, targeted therapies, or traditional cytotoxic or radiation therapy. With the combination of both immune and non-immune treatment approaches, the complexity in making the diagnosis of cardiotoxicity related to an ICI will increase substantially. Here, we summarize the published data on the epidemiology, diagnosis, and management of cardiotoxicity of ICIs. This is a rapidly evolving field, and as our understanding continues to evolve, previously considered hypotheses may not prove to be entirely correct. Research and continued collaborations are urgently needed to provide evidence-based cardiovascular care for this rapidly expanding and vulnerable cohort of patients. (J Am Coll Cardiol CardioOnc 2021;3:35-47) © 2021 The Authors. Published by Elsevier on behalf of the American College of Cardiology Foundation. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords: diagnosis; epidemiology; immunotherapy; myocarditis; treatment.
Conflict of interest statement
Dr. Neilan has received advisory fees from Parexel, Bristol Myers Squibb, H3 Biomedicine, AbbVie, and Intrinsic Imaging. Dr. Lyon has received speaker, advisory board or consultancy fees, and/or research grants from Pfizer, Novartis, Servier, Amgen, Takeda, Roche, Janssens-Cilag Ltd, Clinigen Group, Eli Lily, Eisai, Bristol Myers Squibb, Ferring Pharmaceuticals, and Boehringer Ingelheim. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


References
-
- Reck M., Rodríguez-Abreu D., Robinson A.G. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

