Pathogenicity and virulence of West Nile virus revisited eight decades after its first isolation
- PMID: 33843445
- PMCID: PMC8043182
- DOI: 10.1080/21505594.2021.1908740
Pathogenicity and virulence of West Nile virus revisited eight decades after its first isolation
Abstract
West Nile virus (WNV) is a flavivirus which transmission cycle is maintained between mosquitoes and birds, although it occasionally causes sporadic outbreaks in horses and humans that can result in serious diseases and even death. Since its first isolation in Africa in 1937, WNV had been considered a neglected pathogen until its recent spread throughout Europe and the colonization of America, regions where it continues to cause outbreaks with severe neurological consequences in humans and horses. Although our knowledge about the characteristics and consequences of the virus has increased enormously lately, many questions remain to be resolved. Here, we thoroughly update our knowledge of different aspects of the WNV life cycle: virology and molecular classification, host cell interactions, transmission dynamics, host range, epidemiology and surveillance, immune response, clinical presentations, pathogenesis, diagnosis, prophylaxis (antivirals and vaccines), and prevention, and we highlight those aspects that are still unknown and that undoubtedly require further investigation.
Keywords: West Nile virus; antivirals; clinical presentation; diagnosis; epidemiology; immune response; molecular biology; pathogenesis; transmission; vaccines.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

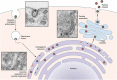

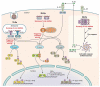
References
-
- Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3(1):13–22. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
