Pathogenicity and virulence of Clostridium perfringens
- PMID: 33843463
- PMCID: PMC8043184
- DOI: 10.1080/21505594.2021.1886777
Pathogenicity and virulence of Clostridium perfringens
Abstract
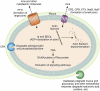
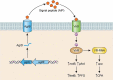
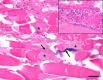
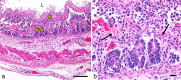
Clostridium perfringens is an extremely versatile pathogen of humans and livestock, causing wound infections like gas gangrene (clostridial myonecrosis), enteritis/enterocolitis (including one of the most common human food-borne illnesses), and enterotoxemia (where toxins produced in the intestine are absorbed and damage distant organs such as the brain). The virulence of this Gram-positive, spore-forming, anaerobe is largely attributable to its copious toxin production; the diverse actions and roles in infection of these toxins are now becoming established. Most C. perfringens toxin genes are encoded on conjugative plasmids, including the pCW3-like and the recently discovered pCP13-like plasmid families. Production of C. perfringens toxins is highly regulated via processes involving two-component regulatory systems, quorum sensing and/or sporulation-related alternative sigma factors. Non-toxin factors, such as degradative enzymes like sialidases, are also now being implicated in the pathogenicity of this bacterium. These factors can promote toxin action in vitro and, perhaps in vivo, and also enhance C. perfringens intestinal colonization, e.g. NanI sialidase increases C. perfringens adherence to intestinal tissue and generates nutrients for its growth, at least in vitro. The possible virulence contributions of many other factors, such as adhesins, the capsule and biofilms, largely await future study.
Keywords: Clostridium perfringens; enteritis/enterocolitis; enterotoxemia; gas gangrene; quorum sensing; sporulation; toxins; two-component regulatory systems.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

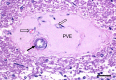

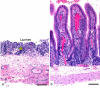
References
-
- McClane BA, Robertson SL, Li J.. Clostridium perfringens. In: Doyle MP, Buchanan RL, editors. In: food Microbiology: fundamentals and Frontiers. 4th ed. ASM press: Washington D.C.; 2013. p. 465–489.
-
- Popoff MR, Bouvet P. Clostridial toxins. Future Microbiol. 2009;4(8):1021–1064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
