Falls Predict Acute Hospitalization in Parkinson's Disease
- PMID: 33843696
- PMCID: PMC9912731
- DOI: 10.3233/JPD-212539
Falls Predict Acute Hospitalization in Parkinson's Disease
Abstract
Background: There is a need for identifying risk factors for hospitalization in Parkinson's disease (PD) and also interventions to reduce acute hospital admission.
Objective: To analyze the frequency, causes, and predictors of acute hospitalization (AH) in PD patients from a Spanish cohort.
Methods: PD patients recruited from 35 centers of Spain from the COPPADIS-2015 (COhort of Patients with PArkinson's DIsease in Spain, 2015) cohort from January 2016 to November 2017, were included in the study. In order to identify predictors of AH, Kaplan-Meier estimates of factors considered as potential predictors were obtained and Cox regression performed on time to hospital encounter 1-year after the baseline visit.
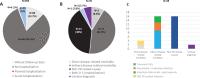
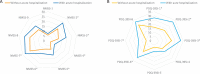
Results: Thirty-five out of 605 (5.8%) PD patients (62.5±8.9 years old; 59.8% males) presented an AH during the 1-year follow-up after the baseline visit. Traumatic falls represented the most frequent cause of admission, being 23.7% of all acute hospitalizations. To suffer from motor fluctuations (HR [hazard ratio] 2.461; 95% CI, 1.065-5.678; p = 0.035), a very severe non-motor symptoms burden (HR [hazard ratio] 2.828; 95% CI, 1.319-6.063; p = 0.008), falls (HR 3.966; 95% CI 1.757-8.470; p = 0.001), and dysphagia (HR 2.356; 95% CI 1.124-4.941; p = 0.023) was associated with AH after adjustment to age, gender, disease duration, levodopa equivalent daily dose, total number of non-antiparkinsonian drugs, and UPDRS-IIIOFF. Of the previous variables, only falls (HR 2.998; 95% CI 1.080-8.322; p = 0.035) was an independent predictor of AH.
Conclusion: Falls is an independent predictor of AH in PD patients.
Conflict of interest statement
Santos García D. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, and Teva.
de Deus Fonticoba T: None.
Cores C. has received honoraria for educational presentations and advice service by Lundbeck and UCB Pharma.
Suárez Castro E: None.
Hernández Vara J. has received honoraria for advice service from Britannia, travel bursaries and educational grants from Abbvie, and has received honoraria for educational presentations from Abbvie, Teva, Bial, Zambon, Italfarmaco, and Sanofi-Genzyme.
Jesús S. has received honoraria from AbbVie, Bial, Merz, UCB, and Zambon and holds the competitive contract “Juan Rodés” supported by the Instituto de Salud Carlos III. She has received grants from the Spanish Ministry of Economy and Competitiveness (PI18/01898) and the Consejería de Salud de la Junta de Andalucía (PI-0459-2018).
Mir P. has received honoraria from AbbVie, Abbott, Allergan, Bial, Merz, UCB, and Zambon. He has received grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Subdirección General de Evaluación y Fomento de la Investigación) and by Fondo Europeo de Desarrollo Regional (FEDER), the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía [CVI-02526, CTS-7685], the Consejería de Salud y Bienestar Social de la Junta de Andalucía [PI-0437-2012, PI-0471-2013], the Sociedad Andaluza de Neu-rología, the Jacques and Gloria Gossweiler Foundation, the Fundación Alicia Koplowitz, and the Funda-ción Mutua Madrileña.
Cosgaya M: None.
Martí MJ. received honoraria for advice and lecture from Abbvie, Bial, and Merzt Pharma and grants from Michael J. Fox Foundation for Parkinson Disease (MJFF): MJF_PPMI_10_001, PI044024; Fondo de Investigacuiones Sanitarias of Spain (FIS PI17/00096) and from Generalitat de Catalunya (AGAUR Exp 2017 SGR 1502).
Pastor P: None.
Cabo I. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
Seijo M. has received honoraria for educational services from KRKA, UCB, Zambon, and Bial; travel grants from Daiichi and Roche.
Legarda I. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva.
Vives B: None.
Caballol N. has received honoraria from Bial, Italfármaco, Qualigen, Zambon, UCB, Teva, and KRKA and sponsorship from Zambon, TEVA, and Abbvie for attending medical conferences.
Rúiz Martínez J. has received honoraria for educational presentations, attending medical conferences, and advice service by Abbvie, UCB Pharma, Zambon, Italfarmaco, Bial, and Teva.
Croitoru I: None.
Cubo E: Travel grants: Abbvie, Allergan, Boston; Lecturing honoraria: Abbvie, International Parkinson’s disease Movement Disorder Society.
Miranda J: None.
Alonso Losada MG. has received honoraria for educational presentations and advice service by Zambon and Bial.
Labandeira C. has received honoraria for educational presentations and advice service by Abbvie, Italfarmaco, Zambon, and Bial.
López Ariztegui N. has received honoraria for educational presentations and advice service by Abbvie, Italfarmaco, Zambon, and Bial.
Morales-Casado M. has received honoraria for educational presentations and advice service by Bial, Zambon, UCB, Ferrer and Fressenius-kabi.
González Aramburu I: None.
Infante J. has received travel bursaries and honoraria for educational presentations from Abbvie and Zambon.
Escalante S. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
Bernardo N: None.
Blázquez Estrada M. has received honoraria for educational presentations and advice service by Abbvie, Abbott, UCB Pharma, Allergan, Zambon, Bial, and Qualigen.
Menéndez M. has received honoraria for educational presentations by KRKA and Zambon.
Seijo M. has received honoraria for educational services from KRKA, UCB, Zambon, and Bial; travel grants from Daiichi and Roche.
García Caldentey J. has received honoraria for educational presentations and advice service by Qualigen, Nutricia, Abbvie, Italfarmaco, UCB Pharma, Lundbeck, Zambon, Bial, and Teva.
Borrué C: None.
Vela L. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva.
Catalán MJ: None.
Gómez-Mayordomo V: None.
Kurtis M. has received honoraria from Bial, the Spanish Neurology Society and the International and Movement Disorders Society.
Prieto C: None.
Ordás C: None.
Nogueira V: None.
López Manzanares L: Compensated advisory services, consulting, research grant support, or speaker honoraria: AbbVie, Acorda, Bial, Intec Pharma, Italfarmaco, Pfizer, Roche, Teva, UCB, and Zambon.
Ávila Rivera MA. has received honoraria from Zambon, UCB Pharma, Qualigen, Bial, and Teva, and sponsorship from Zambon and Teva for attending conferences.
Puente V. has served as consultant for Abbvie and Zambon; has received grant/research from Abbvie.
García Moreno JM. has received honoraria for educational presentations and advice service by Abbvie, Ital-Pharma, Lundbeck, Merz, KRKA, UCB, Pharma, Zambon, Bial and Teva.
Solano Vila B. has received honoraria for educational presentations and advice service by UCB, Zambon, Teva, Abbvie, and Bial.
Álvarez Sauco M. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva.
Carrillo Padilla F. has received honoraria from Zambon (SEN Congress assistance).
Martínez Castrillo JC. has received research support from Lundbeck, Italfarmaco, Allergan, Zambon, Merz, and Abbvie. He has also received speaking honoraria from AbbVie, Bial, Italfarmaco, Lundbeck, Krka, TEVA, UCB, Zambon, Allergan, Ipsen, and Merz.
Sánchez Alonso P. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva.
Gastón I. has received research support from Abbvie and Zambon and has served as a consultant for Abbvie, Exelts, and Zambon.
Kulisevsky J: (1) Consulting fees: Roche, Zambon; (2) Stock / allotment: No; (3) Patent royalties / licensing fees: No; (4) Honoraria (e.g., lecture fees): Zambon, Teva, Bial, UCB; (5) Fees for promotional materials: No; (6) Research funding: Roche, Zambon, Ciberned; Instituto de SaludCarlos III; FundacióLa Maratóde TV3; (7) Scholarship from corporation: No; (8) Corporate laboratory funding: No; (9) Others (e.g., trips, travel, or gifts): No.
Valero C. has received honoraria for educational services from Zambon, Abbvie and UCB.
de Fábregues O. has received honoraria for educational presentations and advice service by Bial, Zambon, Abbvie, KRKA, and Teva.
González Ardura J. has recieved honoraria for speking from italofarma, Krka, Genzyme, UCB, Esteve, Psyma iberica marketing research SL and Ferrer, course grant from Teva and travel grant from Merck.
López Díaz L. has received honoraria from UCB, Lundbeck and KRKA.
Martinez-Martin P. has received honoraria from Editorial Viguera and Takeda Pharmaceuticals for lecturing in courses; from Britannia for writing an article in their Parkinson’s Disease Medical Journal-Kinetic; and from the International Parkinson and Movement Disorder Society (MDS) for management of the Program on Rating Scales. Grants from the MDS for development and validation of the MDS-NMS.
Figures


References
-
- Guttman M, Slaughter PM, Theriault ME, DeBoer DP, Naylor CD (2003) Burden of parkinsonism: A population-based study, Mov Disord 18, 313–319. - PubMed
-
- Aminoff MJ, Christine CW, Friedman JH, Chou KL, Lyons KE, Pahwa R, Bloem BR, Parashos SA, Price CC, Malaty IA, Iansek R, Bodis-Wollner I, Suchowersky O, Oertel WH, Zamudio J, Oberdorf J, Schmidt P, Okun MS; National Parkinson Foundation Working Group on Hospitalization in Parkinson’s Disease (2011) National Parkinson Foundation Working Group on Hospitalization in Parkinson’s Disease. Management of the hospitalized patient with Parkinson’s disease: Current state of the field and need for guidelines, Parkinsonism Relat Disord 17, 139–145. - PMC - PubMed
-
- Woodford H, Walker R (2005) Emergency hospital admissions in idiopathic Parkinson’s disease, Mov Disord 20, 1104–1108. - PubMed
-
- Low V, Ben-Shlomo Y, Coward E, Fletcher S, Walker R, Clarke CE (2015) Measuring the burden and mortality of hospitalisation in Parkinson’s disease: A cross-sectional analysis of the English Hospital Episodes Statistics database 2009-2013, Parkinsonism Relat Disord 21, 449–454. - PubMed
-
- Ahlskog JE (2014) Parkinson disease treatment in hospitals and nursing facilities: Avoiding pitfalls, Mayo Clin Proc 89, 997–1003. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

