Depletion of mitochondrial inorganic polyphosphate (polyP) in mammalian cells causes metabolic shift from oxidative phosphorylation to glycolysis
- PMID: 33843973
- PMCID: PMC8145922
- DOI: 10.1042/BCJ20200975
Depletion of mitochondrial inorganic polyphosphate (polyP) in mammalian cells causes metabolic shift from oxidative phosphorylation to glycolysis
Abstract
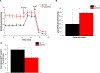
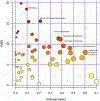
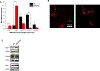
Inorganic polyphosphate (polyP) is a linear polymer composed of up to a few hundred orthophosphates linked together by high-energy phosphoanhydride bonds, identical with those found in ATP. In mammalian mitochondria, polyP has been implicated in multiple processes, including energy metabolism, ion channels function, and the regulation of calcium signaling. However, the specific mechanisms of all these effects of polyP within the organelle remain poorly understood. The central goal of this study was to investigate how mitochondrial polyP participates in the regulation of the mammalian cellular energy metabolism. To accomplish this, we created HEK293 cells depleted of mitochondrial polyP, through the stable expression of the polyP hydrolyzing enzyme (scPPX). We found that these cells have significantly reduced rates of oxidative phosphorylation (OXPHOS), while their rates of glycolysis were elevated. Consistent with this, metabolomics assays confirmed increased levels of metabolites involved in glycolysis in these cells, compared with the wild-type samples. At the same time, key respiratory parameters of the isolated mitochondria were unchanged, suggesting that respiratory chain activity is not affected by the lack of mitochondrial polyP. However, we detected that mitochondria from cells that lack mitochondrial polyP are more fragmented when compared with those from wild-type cells. Based on these results, we propose that mitochondrial polyP plays an important role as a regulator of the metabolic switch between OXPHOS and glycolysis.
Keywords: glycolysis; inorganic polyphosphates; mitochondrial bioenergetics; oxidative phosphorylation; polyP.
© 2021 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
Differential effects of endo- and exopolyphosphatase expression on the induction of the mitochondrial permeability transition pore.Biochim Biophys Acta Biomembr. 2025 Mar;1867(3):184408. doi: 10.1016/j.bbamem.2025.184408. Epub 2025 Jan 7. Biochim Biophys Acta Biomembr. 2025. PMID: 39788473
-
Inorganic polyphosphate and the regulation of mitochondrial physiology.Biochem Soc Trans. 2023 Dec 20;51(6):2153-2161. doi: 10.1042/BST20230735. Biochem Soc Trans. 2023. PMID: 37955101 Free PMC article.
-
Is there a link between inorganic polyphosphate (polyP), mitochondria, and neurodegeneration?Pharmacol Res. 2021 Jan;163:105211. doi: 10.1016/j.phrs.2020.105211. Epub 2020 Oct 1. Pharmacol Res. 2021. PMID: 33010423 Free PMC article. Review.
-
Inorganic polyphosphate is produced and hydrolyzed in F0F1-ATP synthase of mammalian mitochondria.Biochem J. 2020 Apr 30;477(8):1515-1524. doi: 10.1042/BCJ20200042. Biochem J. 2020. PMID: 32270854 Free PMC article.
-
Inorganic polyphosphates in mitochondria.Biochemistry (Mosc). 2010 Jul;75(7):825-31. doi: 10.1134/s0006297910070035. Biochemistry (Mosc). 2010. PMID: 20673205 Review.
Cited by
-
Inorganic Polyphosphate: Coacervate Formation and Functional Significance in Nanomedical Applications.Int J Nanomedicine. 2022 Nov 30;17:5825-5850. doi: 10.2147/IJN.S389819. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36474526 Free PMC article. Review.
-
Therapeutic potential of chrysin nanoparticle-mediation inhibition of succinate dehydrogenase and ubiquinone oxidoreductase in pancreatic and lung adenocarcinoma.Eur J Med Res. 2022 Sep 8;27(1):172. doi: 10.1186/s40001-022-00803-y. Eur J Med Res. 2022. PMID: 36076266 Free PMC article. Review.
-
Mitochondrial inorganic polyphosphate is required to maintain proteostasis within the organelle.Front Cell Dev Biol. 2024 Jul 10;12:1423208. doi: 10.3389/fcell.2024.1423208. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39050895 Free PMC article.
-
The deubiquitinase Ubp3/Usp10 constrains glucose-mediated mitochondrial repression via phosphate budgeting.Elife. 2024 Sep 26;12:RP90293. doi: 10.7554/eLife.90293. Elife. 2024. PMID: 39324403 Free PMC article.
-
The Glacier Ice Worm, Mesenchytraeus solifugus, Elevates Mitochondrial Inorganic Polyphosphate (PolyP) Levels in Response to Stress.Biology (Basel). 2022 Dec 6;11(12):1771. doi: 10.3390/biology11121771. Biology (Basel). 2022. PMID: 36552279 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

