Distinctive clinical presentation and pathogenic specificities of anti-AK5 encephalitis
- PMID: 33843981
- PMCID: PMC8557339
- DOI: 10.1093/brain/awab153
Distinctive clinical presentation and pathogenic specificities of anti-AK5 encephalitis
Abstract
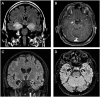
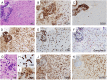
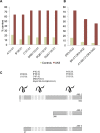
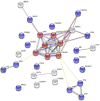
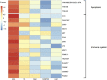
Limbic encephalitis with antibodies against adenylate kinase 5 (AK5) has been difficult to characterize because of its rarity. In this study, we identified 10 new cases and reviewed 16 previously reported patients, investigating clinical features, IgG subclasses, human leucocyte antigen and CSF proteomic profiles. Patients with anti-AK5 limbic encephalitis were mostly male (20/26, 76.9%) with a median age of 66 years (range 48-94). The predominant symptom was severe episodic amnesia in all patients, and this was frequently associated with depression (17/25, 68.0%). Weight loss, asthenia and anorexia were also highly characteristic, being present in 11/25 (44.0%) patients. Although epilepsy was always lacking at disease onset, seizures developed later in a subset of patients (4/25, 16.0%). All patients presented CSF abnormalities, such as pleocytosis (18/25, 72.0%), oligoclonal bands (18/25, 72.0%) and increased Tau (11/14, 78.6%). Temporal lobe hyperintensities were almost always present at disease onset (23/26, 88.5%), evolving nearly invariably towards severe atrophy in subsequent MRIs (17/19, 89.5%). This finding was in line with a poor response to immunotherapy, with only 5/25 (20.0%) patients responding. IgG1 was the predominant subclass, being the most frequently detected and the one with the highest titres in nine CSF-serum paired samples. A temporal biopsy from one of our new cases showed massive lymphocytic infiltrates dominated by both CD4+ and CT8+ T cells, intense granzyme B expression and abundant macrophages/microglia. Human leucocyte antigen (HLA) analysis in 11 patients showed a striking association with HLA-B*08:01 [7/11, 63.6%; odds ratio (OR) = 13.4, 95% confidence interval (CI): 3.8-47.4], C*07:01 (8/11, 72.7%; OR = 11.0, 95% CI: 2.9-42.5), DRB1*03:01 (8/11, 72.7%; OR = 14.4, 95% CI: 3.7-55.7), DQB1*02:01 (8/11, 72.7%; OR = 13.5, 95% CI: 3.5-52.0) and DQA1*05:01 (8/11, 72.7%; OR = 14.4, 95% CI: 3.7-55.7) alleles, which formed the extended haplotype B8-C7-DR3-DQ2 in 6/11 (54.5%) patients (OR = 16.5, 95% CI: 4.8-57.1). Finally, we compared the CSF proteomic profile of five anti-AK5 patients with that of 40 control subjects and 10 cases with other more common non-paraneoplastic limbic encephalitis (five with antibodies against leucine-rich glioma inactivated 1 and five against contactin-associated protein-like 2), as well as 10 cases with paraneoplastic neurological syndromes (five with antibodies against Yo and five against Ma2). These comparisons revealed 31 and seven significantly upregulated proteins in anti-AK5 limbic encephalitis, respectively mapping to apoptosis pathways and innate/adaptive immune responses. These findings suggest that the clinical manifestations of anti-AK5 limbic encephalitis result from a distinct T cell-mediated pathogenesis, with major cytotoxicity-induced apoptosis leading to a prompt and aggressive neuronal loss, likely explaining the poor prognosis and response to immunotherapy.
Keywords: adenylate kinase 5; human leucocyte antigen; limbic encephalitis; proteomics.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Do L-D, Chanson E, Desestret V, et al.Characteristics in limbic encephalitis with anti–adenylate kinase 5 autoantibodies. Neurology. 2017;88(6):514–524. - PubMed
-
- Bien CI, Nehls F, Kollmar R, et al.Identification of adenylate kinase 5 antibodies during routine diagnostics in a tissue-based assay: Three new cases and a review of the literature. J Neuroimmunol. 2019;334:576975. - PubMed
-
- Zoccarato M, Valeggia S, Zuliani L, et al.Conventional brain MRI features distinguishing limbic encephalitis from mesial temporal glioma. Neuroradiology. 2019;61(8):853–860. - PubMed
-
- Vogrig A, Joubert B, André‐Obadia N, Gigli GL, Rheims S, Honnorat J.. Seizure specificities in patients with antibody‐mediated autoimmune encephalitis. Epilepsia. 2019;60(8):1508–1525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

