Temozolomide with irinotecan versus temozolomide, irinotecan plus bevacizumab for recurrent medulloblastoma of childhood: Report of a COG randomized Phase II screening trial
- PMID: 33844469
- PMCID: PMC8764558
- DOI: 10.1002/pbc.29031
Temozolomide with irinotecan versus temozolomide, irinotecan plus bevacizumab for recurrent medulloblastoma of childhood: Report of a COG randomized Phase II screening trial
Abstract
Background: Approximately 30% of children with medulloblastoma (MB) experience recurrence, which is usually incurable. This study compared the overall survival (OS) of patients receiving temozolomide (TMZ) and irinotecan with that of patients receiving TMZ, irinotecan, and bevacizumab for recurrent MB/central nervous system (CNS) primitive neuroectodermal tumor (PNET).
Methods: Patients with relapsed/refractory MB or CNS PNET were randomly assigned to receive TMZ (150 mg/m2 /day PO on days 1-5) and irinotecan (50 mg/m2 /day IV on days 1-5) with or without bevacizumab (10 mg/kg IV on days 1 and 15).
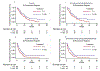
Results: One hundred five patients were eligible and treated on study. Median OS was 13 months in the standard arm and 19 months with the addition of bevacizumab; median event-free survival (EFS) was 6 months in the standard arm and 9 months with the addition of bevacizumab. The hazard ratio for death from the stratified relative-risk regression model is 0.63. Overall, 23 patients completed 12 courses of planned protocol therapy, 23% (12/52) in the experimental arm with bevacizumab versus 21% (11/53) in the standard arm. Toxicity profiles were comparable in both treatment arms. The estimate of the incidence of feasibility events associated with the bevacizumab arm is three of 52 (5.8%) (95% CI 1.2-16%). Events included myelosuppression, electrolyte abnormalities, diarrhea, and elevated transaminases. One intracranial hemorrhage event was observed in each arm.
Conclusion: The addition of bevacizumab to TMZ/irinotecan significantly reduced the risk of death in children with recurrent MB. The combination was relatively well tolerated in this heavily pretreated cohort. The three-drug regimen demonstrated a sufficient risk reduction to warrant further investigation.
Keywords: PNET; bevacizumab; irinotecan; recurrent medulloblastoma; temozolomide.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Figures


References
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D6, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016. Jun;131(6):803–20. doi: 10.1007/s00401-016-1545-1. Epub 2016 May 9. DOI:10.1007/s00401-016-1545-1 - DOI - DOI - PubMed
-
- Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, Kool M7, Dufour C, Vassal G, Milde T, Witt O, von Hoff K, Pietsch T, Northcott PA 13, Gajjar A, Robinson GW, Padovani L, André N, Massimino M, Pizer B, Packer R, Rutkowski S, Pfister SM, Taylor MD, Pomeroy SL. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016. Jun;131(6):821–31. doi: 10.1007/s00401-016-1569-6. Epub 2016 Apr 4.. DOI: 10.1007/s00401-016-1569-6. - DOI - DOI - PMC - PubMed
-
- Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape. J Clin Oncol. 2015. Sep 20;33(27):2986–98. doi: 10.1200/JCO.2014.59.9217. Epub 2015 Aug 24. DOI: 10.1200/JCO.2014.59.9217 - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

