Profiling the Tox21 Chemical Collection for Acetylcholinesterase Inhibition
- PMID: 33844597
- PMCID: PMC8041433
- DOI: 10.1289/EHP6993
Profiling the Tox21 Chemical Collection for Acetylcholinesterase Inhibition
Abstract
Background: Inhibition of acetylcholinesterase (AChE), a biomarker of organophosphorous and carbamate exposure in environmental and occupational human health, has been commonly used to identify potential safety liabilities. So far, many environmental chemicals, including drug candidates, food additives, and industrial chemicals, have not been thoroughly evaluated for their inhibitory effects on AChE activity. AChE inhibitors can have therapeutic applications (e.g., tacrine and donepezil) or neurotoxic consequences (e.g., insecticides and nerve agents).
Objectives: The objective of the current study was to identify environmental chemicals that inhibit AChE activity using in vitro and in silico models.
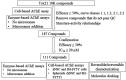
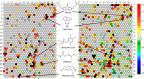
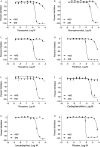
Methods: To identify AChE inhibitors rapidly and efficiently, we have screened the Toxicology in the 21st Century (Tox21) 10K compound library in a quantitative high-throughput screening (qHTS) platform by using the homogenous cell-based AChE inhibition assay and enzyme-based AChE inhibition assays (with or without microsomes). AChE inhibitors identified from the primary screening were further tested in monolayer or spheroid formed by SH-SY5Y and neural stem cell models. The inhibition and binding modes of these identified compounds were studied with time-dependent enzyme-based AChE inhibition assay and molecular docking, respectively.
Results: A group of known AChE inhibitors, such as donepezil, ambenonium dichloride, and tacrine hydrochloride, as well as many previously unreported AChE inhibitors, such as chelerythrine chloride and cilostazol, were identified in this study. Many of these compounds, such as pyrazophos, phosalone, and triazophos, needed metabolic activation. This study identified both reversible (e.g., donepezil and tacrine) and irreversible inhibitors (e.g., chlorpyrifos and bromophos-ethyl). Molecular docking analyses were performed to explain the relative inhibitory potency of selected compounds.
Conclusions: Our tiered qHTS approach allowed us to generate a robust and reliable data set to evaluate large sets of environmental compounds for their AChE inhibitory activity. https://doi.org/10.1289/EHP6993.
Figures
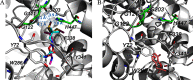
References
-
- Abass K, Turpeinen M, Rautio A, Hakkola J, Pelkonen O. 2011. Metabolism of pesticides by human cytochrome P450 enzymes in vitro - a survey. In: Insecticides - Advances in Integrated Pest Management Perveen FK, ed. InTech, 165–194, 10.13140/2.1.3501.5689. - DOI
-
- Almasieh M, MacIntyre JN, Pouliot M, Casanova C, Vaucher E, Kelly ME, et al. . 2013. Acetylcholinesterase inhibition promotes retinal vasoprotection and increases ocular blood flow in experimental glaucoma. Invest Ophthalmol Vis Sci 54(5):3171–3183, PMID: 23599333, 10.1167/iovs.12-11481. - DOI - PubMed
-
- Arya S, Kumar A, Kumar N, Roy P, Sondhi SM. 2015. Synthesis and anticancer activity evaluation of some acridine derivatives. Med Chem Res 24(5):1942–1951, 10.1007/s00044-014-1268-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

