Highs and Lows of Bond Lengths: Is There Any Limit?
- PMID: 33844880
- PMCID: PMC8362100
- DOI: 10.1002/anie.202102967
Highs and Lows of Bond Lengths: Is There Any Limit?
Abstract
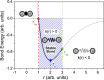
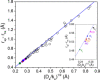
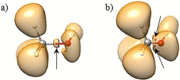
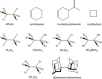
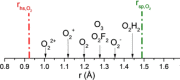
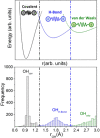
Two distinct points on the potential energy curve (PEC) of a pairwise interaction, the zero-energy crossing point and the point where the stretching force constant vanishes, allow us to anticipate the range of possible distances between two atoms in diatomic, molecular moieties and crystalline systems. We show that these bond-stability boundaries are unambiguously defined and correlate with topological descriptors of electron-density-based scalar fields, and can be calculated using generic PECs. Chemical databases and quantum-mechanical calculations are used to analyze a full set of diatomic bonds of atoms from the s-p main block. Emphasis is placed on the effect of substituents in C-C covalent bonds, concluding that distances shorter than 1.14 Å or longer than 2.0 Å are unlikely to be achieved, in agreement with ultra-high-pressure data and transition-state distances, respectively. Presumed exceptions are used to place our model in the correct framework and to formulate a conjecture for chained interactions, which offers an explanation for the multimodal histogram of O-H distances reported for hundreds of chemical systems.
Keywords: ELF (electron localization function); bond length; carbon-carbon bonds; hydrogen bonds; potential energy curve.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Pauling L., The nature of the chemical bond and the structure of molecules and crystals: An introduction to modern structural chemistry, University Press, Ithaca, 1960.
-
- Kaupp M., Danovich D., Shaik S., Coord. Chem. Rev. 2017, 344, 355–362.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

