SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN)
- PMID: 33844963
- PMCID: PMC8040523
- DOI: 10.1016/S0140-6736(21)00675-9
SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN)
Erratum in
-
Department of Error.Lancet. 2021 May 8;397(10286):1710. doi: 10.1016/S0140-6736(21)01009-6. Lancet. 2021. PMID: 33965089 Free PMC article. No abstract available.
Abstract
Background: Increased understanding of whether individuals who have recovered from COVID-19 are protected from future SARS-CoV-2 infection is an urgent requirement. We aimed to investigate whether antibodies against SARS-CoV-2 were associated with a decreased risk of symptomatic and asymptomatic reinfection.
Methods: A large, multicentre, prospective cohort study was done, with participants recruited from publicly funded hospitals in all regions of England. All health-care workers, support staff, and administrative staff working at hospitals who could remain engaged in follow-up for 12 months were eligible to join The SARS-CoV-2 Immunity and Reinfection Evaluation study. Participants were excluded if they had no PCR tests after enrolment, enrolled after Dec 31, 2020, or had insufficient PCR and antibody data for cohort assignment. Participants attended regular SARS-CoV-2 PCR and antibody testing (every 2-4 weeks) and completed questionnaires every 2 weeks on symptoms and exposures. At enrolment, participants were assigned to either the positive cohort (antibody positive, or previous positive PCR or antibody test) or negative cohort (antibody negative, no previous positive PCR or antibody test). The primary outcome was a reinfection in the positive cohort or a primary infection in the negative cohort, determined by PCR tests. Potential reinfections were clinically reviewed and classified according to case definitions (confirmed, probable, or possible) and symptom-status, depending on the hierarchy of evidence. Primary infections in the negative cohort were defined as a first positive PCR test and seroconversions were excluded when not associated with a positive PCR test. A proportional hazards frailty model using a Poisson distribution was used to estimate incidence rate ratios (IRR) to compare infection rates in the two cohorts.
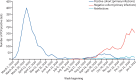
Findings: From June 18, 2020, to Dec 31, 2020, 30 625 participants were enrolled into the study. 51 participants withdrew from the study, 4913 were excluded, and 25 661 participants (with linked data on antibody and PCR testing) were included in the analysis. Data were extracted from all sources on Feb 5, 2021, and include data up to and including Jan 11, 2021. 155 infections were detected in the baseline positive cohort of 8278 participants, collectively contributing 2 047 113 person-days of follow-up. This compares with 1704 new PCR positive infections in the negative cohort of 17 383 participants, contributing 2 971 436 person-days of follow-up. The incidence density was 7·6 reinfections per 100 000 person-days in the positive cohort, compared with 57·3 primary infections per 100 000 person-days in the negative cohort, between June, 2020, and January, 2021. The adjusted IRR was 0·159 for all reinfections (95% CI 0·13-0·19) compared with PCR-confirmed primary infections. The median interval between primary infection and reinfection was more than 200 days.
Interpretation: A previous history of SARS-CoV-2 infection was associated with an 84% lower risk of infection, with median protective effect observed 7 months following primary infection. This time period is the minimum probable effect because seroconversions were not included. This study shows that previous infection with SARS-CoV-2 induces effective immunity to future infections in most individuals.
Funding: Department of Health and Social Care of the UK Government, Public Health England, The National Institute for Health Research, with contributions from the Scottish, Welsh and Northern Irish governments.
Crown copyright © 2021 Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
A New Normal Once Again.Pediatr Ann. 2021 Jul;50(7):e268-e269. doi: 10.3928/19382359-20210617-02. Epub 2021 Jul 1. Pediatr Ann. 2021. PMID: 34264795 No abstract available.
Dataset use reported in
-
Correlates of protection from SARS-CoV-2 infection.Lancet. 2021 Apr 17;397(10283):1421-1423. doi: 10.1016/S0140-6736(21)00782-0. Epub 2021 Apr 9. Lancet. 2021. PMID: 33844964 Free PMC article. No abstract available.
References
-
- European Centre for Disease Prevention and Control Reinfection with SARS-CoV: considerations for public health response. 2020. https://www.ecdc.europa.eu/sites/default/files/documents/Re-infection-an...
-
- Selhorst P, Van Ierssel S, Michiels J, et al. Symptomatic SARS-CoV-2 re-infection of a health care worker in a Belgian nosocomial outbreak despite primary neutralizing antibody response. medRxiv. 2020 https://www.medrxiv.org/content/10.1101/2020.11.05.20225052v1 published online Nov 9. (preprint). - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

