Methadone and buprenorphine discontinuation among postpartum women with opioid use disorder
- PMID: 33845029
- PMCID: PMC8492487
- DOI: 10.1016/j.ajog.2021.04.210
Methadone and buprenorphine discontinuation among postpartum women with opioid use disorder
Abstract
Background: The postpartum year is a vulnerable period for women with opioid use disorder, with increased rates of fatal and nonfatal overdose; however, data on the continuation of medications for opioid use disorder on a population level are limited.
Objective: This study aimed to examine the effect of discontinuing methadone and buprenorphine in women with opioid use disorder in the year following delivery and determine the extent to which maternal and infant characteristics are associated with time to discontinuation of medications for opioid use disorder.
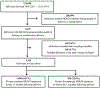
Study design: This population-based retrospective cohort study used linked administrative data of 211,096 deliveries in Massachusetts between 2011 and 2014 to examine the adherence to medications for opioid use disorder. Individuals receiving medications for opioid use disorder after delivery were included in the study. Here, demographic, psychosocial, prenatal, and delivery characteristics are described. Kaplan-Meier survival analysis and Cox regression modeling were used to examine factors associated with medication discontinuation.
Results: A total of 2314 women who received medications for opioid use disorder at delivery were included in our study. Overall, 1484 women (64.1%) continued receiving medications for opioid use disorder for a full 12 months following delivery. The rate of continued medication use varied from 34% if women started on medications for opioid use disorder the month before delivery to 80% if the medications were used throughout pregnancy. Kaplan-Meier survival curves differed by maternal race and ethnicity (the 12-month continuation probability was .65 for White non-Hispanic women and .51 for non-White women; P<.001) and duration of use of prenatal medications for opioid use disorder (12-month continuation probability was .78 for women with full prenatal engagement and .60 and .44 for those receiving medications for opioid use disorder ≥5 months [but not throughout pregnancy] and ≤4 months prenatally, respectively; P<.001). In all multivariable models, duration of receipt of prenatal medications for opioid use disorder (≤4 months vs throughout pregnancy: adjusted hazard ratio, 3.26; 95% confidence interval, 2.72-3.91) and incarceration (incarceration during pregnancy or after delivery vs none: adjusted hazard ratio, 1.79; 95% confidence interval, 1.52-2.12) were most strongly associated with the discontinuation of medications for opioid use disorder.
Conclusion: Almost two-thirds of women with opioid use disorder continued using medications for opioid use disorder for a full year after delivery; however, the rates of medication continuation varied significantly by race and ethnicity, degree of use of prenatal medications for opioid use disorder, and incarceration status. Prioritizing medication continuation across the perinatal continuum, enhancing sex-specific and family-friendly recovery supports, and expanding access to medications for opioid use disorder despite being incarcerated can help improve postpartum medication adherence.
Keywords: adherence; buprenorphine; discontinuation; disparities; medication for drug use disorder; methadone; opioid use disorder; perinatal continuum; postpartum; substance use disorder.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
References
-
- Hogan L, Rutherford BK, Schrader DR. Maryland Maternal Mortality Review 2016 Annual Report Health – General Article 13-1203–1207.
-
- Kavanaugh V Pregnancy-Associated Deaths From Drug Overdose in Virginia, 1999-2007: A Report from the Virginia Maternal Mortality Review Team.; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical