Measuring compartmental T2-orientational dependence in human brain white matter using a tiltable RF coil and diffusion-T2 correlation MRI
- PMID: 33845062
- PMCID: PMC8270891
- DOI: 10.1016/j.neuroimage.2021.117967
Measuring compartmental T2-orientational dependence in human brain white matter using a tiltable RF coil and diffusion-T2 correlation MRI
Abstract
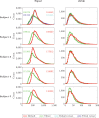
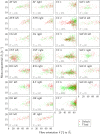
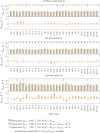
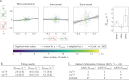
The anisotropy of brain white matter microstructure manifests itself in orientational-dependence of various MRI contrasts, and can result in significant quantification biases if ignored. Understanding the origins of this orientation-dependence could enhance the interpretation of MRI signal changes in development, ageing and disease and ultimately improve clinical diagnosis. Using a novel experimental setup, this work studies the contributions of the intra- and extra-axonal water to the orientation-dependence of one of the most clinically-studied parameters, apparent transverse relaxation T2. Specifically, a tiltable receive coil is interfaced with an ultra-strong gradient MRI scanner to acquire multidimensional MRI data with an unprecedented range of acquisition parameters. Using this setup, compartmental T2 can be disentangled based on differences in diffusional-anisotropy, and its orientation-dependence further elucidated by re-orienting the head with respect to the main magnetic field B→0. A dependence of (compartmental) T2 on the fibre orientation w.r.t. B→0 was observed, and further quantified using characteristic representations for susceptibility- and magic angle effects. Across white matter, anisotropy effects were dominated by the extra-axonal water signal, while the intra-axonal water signal decay varied less with fibre-orientation. Moreover, the results suggest that the stronger extra-axonal T2 orientation-dependence is dominated by magnetic susceptibility effects (presumably from the myelin sheath) while the weaker intra-axonal T2 orientation-dependence may be driven by a combination of microstructural effects. Even though the current design of the tiltable coil only offers a modest range of angles, the results demonstrate an overall effect of tilt and serve as a proof-of-concept motivating further hardware development to facilitate experiments that explore orientational anisotropy. These observations have the potential to lead to white matter microstructural models with increased compartmental sensitivity to disease, and can have direct consequences for longitudinal and group-wise T2- and diffusion-MRI data analysis, where the effect of head-orientation in the scanner is commonly ignored.
Keywords: relaxation; Diffusion MRI; Directional anisotropy; Microstructure; Myelin susceptibility.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



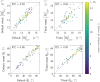
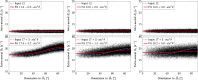
References
-
- Abragam A. Oxford university press; 1961. The Principles of Nuclear Magnetism 32.
-
- Akaike H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974;19(6):716–723.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

