High-salt diet downregulates TREM2 expression and blunts efferocytosis of macrophages after acute ischemic stroke
- PMID: 33845849
- PMCID: PMC8040220
- DOI: 10.1186/s12974-021-02144-9
High-salt diet downregulates TREM2 expression and blunts efferocytosis of macrophages after acute ischemic stroke
Abstract
Background: A high-salt diet (HSD) is one of the major risk factors for acute ischemic stroke (AIS). As a potential mechanism, surplus salt intake primes macrophages towards a proinflammatory phenotype. In this study, whether HSD could blunt the efferocytic capability of macrophages after ischemic stroke, thus exacerbating post-stroke neural inflammation, was investigated.
Methods: Wild-type male C57BL/6 mice were fed with fodder containing 8% sodium chloride for 4 weeks and subjected to transient middle cerebral occlusion (tMCAO). Disease severity, macrophage polarization as well as efferocytic capability were evaluated. Bone marrow-derived macrophages were cultured in vitro, and the impact of high salinity on their efferocytic activity, as well as their expression of phagocytic molecules, were analyzed. The relationships among sodium concentration, macrophage phenotype, and disease severity in AIS patients were explored.
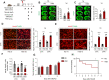
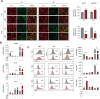
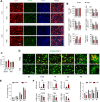
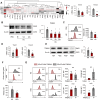
Results: HSD-fed mice displayed increased infarct volume and aggravated neurological deficiency. Mice fed with HSD suffered exacerbated neural inflammation as shown by higher inflammatory mediator expression and immune cell infiltration levels. Infiltrated macrophages within stroke lesions in HSD-fed mice exhibited a shift towards proinflammatory phenotype and impaired efferocytic capability. As assessed with a PCR array, the expression of triggering receptor expressed on myeloid cells 2 (TREM2), a receptor relevant to phagocytosis, was downregulated in high-salt-treated bone marrow-derived macrophages. Enhancement of TREM2 signaling restored the efferocytic capacity and cellular inflammation resolution of macrophages in a high salinity environment in vitro and in vivo. A high concentration of urine sodium in AIS patients was found to be correlated with lower TREM2 expression and detrimental stroke outcomes.
Conclusions: HSD inhibited the efferocytic capacity of macrophages by downregulating TREM2 expression, thus impeding inflammation resolution after ischemic stroke. Enhancing TREM2 signaling in monocytes/macrophages could be a promising therapeutic strategy to enhance efferocytosis and promote post-stroke inflammation resolution.
Keywords: High-salt diet; Macrophage; Phagocytosis; Stroke; Triggering receptor expressed on myeloid cells 2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Triggering receptor expressed on myeloid cells-2 expression in the brain is required for maximal phagocytic activity and improved neurological outcomes following experimental stroke.J Cereb Blood Flow Metab. 2019 Oct;39(10):1906-1918. doi: 10.1177/0271678X18817282. Epub 2018 Dec 7. J Cereb Blood Flow Metab. 2019. PMID: 30523715 Free PMC article.
-
Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke.J Neurosci. 2015 Feb 25;35(8):3384-96. doi: 10.1523/JNEUROSCI.2620-14.2015. J Neurosci. 2015. PMID: 25716838 Free PMC article.
-
Astrocyte-TREM2 alleviates brain injury by regulating reactive astrocyte states following ischemic stroke.Glia. 2024 Nov;72(11):2061-2078. doi: 10.1002/glia.24597. Epub 2024 Jul 26. Glia. 2024. PMID: 39056459
-
TREM2, microglial and ischemic stroke.J Neuroimmunol. 2023 Aug 15;381:578108. doi: 10.1016/j.jneuroim.2023.578108. Epub 2023 May 16. J Neuroimmunol. 2023. PMID: 37302170 Review.
-
The versatile role of TREM2 in regulating of microglia fate in the ischemic stroke.Int Immunopharmacol. 2022 Aug;109:108733. doi: 10.1016/j.intimp.2022.108733. Epub 2022 May 4. Int Immunopharmacol. 2022. PMID: 35525233 Review.
Cited by
-
Inflammatory Responses After Ischemic Stroke.Semin Immunopathol. 2022 Sep;44(5):625-648. doi: 10.1007/s00281-022-00943-7. Epub 2022 Jun 29. Semin Immunopathol. 2022. PMID: 35767089 Review.
-
Efferocytosis: A new therapeutic target for stroke.Chin Med J (Engl). 2024 Dec 5;137(23):2843-2850. doi: 10.1097/CM9.0000000000003363. Epub 2024 Nov 12. Chin Med J (Engl). 2024. PMID: 39528491 Free PMC article. Review.
-
Roles of peripheral immune cells in the recovery of neurological function after ischemic stroke.Front Cell Neurosci. 2022 Oct 21;16:1013905. doi: 10.3389/fncel.2022.1013905. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36339825 Free PMC article. Review.
-
Analysis of diet-related stroke disease burden in China from 1990 to 2021 and projections for 2025-2044.Front Nutr. 2025 Jun 2;12:1571916. doi: 10.3389/fnut.2025.1571916. eCollection 2025. Front Nutr. 2025. PMID: 40525133 Free PMC article.
-
Gas6 Promotes Microglia Efferocytosis and Suppresses Inflammation Through Activating Axl/Rac1 Signaling in Subarachnoid Hemorrhage Mice.Transl Stroke Res. 2023 Dec;14(6):955-969. doi: 10.1007/s12975-022-01099-0. Epub 2022 Nov 3. Transl Stroke Res. 2023. PMID: 36324028
References
-
- Kono Y, Yamada S, Yamaguchi J, Hagiwara Y, Iritani N, Ishida S, et al. Secondary prevention of new vascular events with lifestyle intervention in patients with noncardioembolic mild ischemic stroke: a single-center randomized controlled trial. Cerebrovasc Dis. 2013;36(2):88–97. doi: 10.1159/000352052. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

