Resolvin D1 reduces cancer growth stimulating a protective neutrophil-dependent recruitment of anti-tumor monocytes
- PMID: 33845864
- PMCID: PMC8040222
- DOI: 10.1186/s13046-021-01937-3
Resolvin D1 reduces cancer growth stimulating a protective neutrophil-dependent recruitment of anti-tumor monocytes
Abstract
Background: Innovative therapies to target tumor-associated neutrophils (PMN) are of clinical interest, since these cells are centrally involved in cancer inflammation and tumor progression. Resolvin D1 (RvD1) is a lipid autacoid that promotes resolution of inflammation by regulating the activity of distinct immune and non-immune cells. Here, using human papilloma virus (HPV) tumorigenesis as a model, we investigated whether RvD1 modulates PMN to reduce tumor progression.
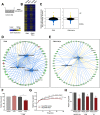
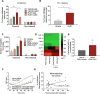
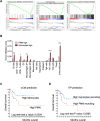
Methods: Growth-curve assays with multiple cell lines and in vivo grafting of two distinct HPV-positive cells in syngeneic mice were used to determine if RvD1 reduced cancer growth. To investigate if and how RvD1 modulates PMN activities, RNA sequencing and multiplex cytokine ELISA of human PMN in co-culture with HPV-positive cells, coupled with pharmacological depletion of PMN in vivo, were performed. The mouse intratumoral immune cell composition was evaluated through FACS analysis. Growth-curve assays and in vivo pharmacological depletion were used to evaluate anti-tumor activities of human and mouse monocytes, respectively. Bioinformatic analysis of The Cancer Genome Atlas (TCGA) database was exploited to validate experimental findings in patients.
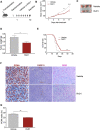
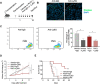
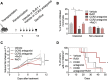
Results: RvD1 decreased in vitro and in vivo proliferation of human and mouse HPV-positive cancer cells through stimulation of PMN anti-tumor activities. In addition, RvD1 stimulated a PMN-dependent recruitment of classical monocytes as key determinant to reduce tumor growth in vivo. In human in vitro systems, exposure of PMN to RvD1 increased the production of the monocyte chemoattractant protein-1 (MCP-1), and enhanced transmigration of classical monocytes, with potent anti-tumor actions, toward HPV-positive cancer cells. Consistently, mining of immune cells infiltration levels in cervical cancer patients from the TCGA database evidenced an enhanced immune reaction and better clinical outcomes in patients with higher intratumoral monocytes as compared to patients with higher PMN infiltration.
Conclusions: RvD1 reduces cancer growth by activating PMN anti-cancer activities and encouraging a protective PMN-dependent recruitment of anti-tumor monocytes. These findings demonstrate efficacy of RvD1 as an innovative therapeutic able to stimulate PMN reprogramming to an anti-cancer phenotype that restrains tumor growth.
Keywords: Classical monocytes; HPV cancers; Neutrophils; Resolution of inflammation; Resolvin D1.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Immunoresolving actions of oral resolvin D1 include selective regulation of the transcription machinery in resolution-phase mouse macrophages.FASEB J. 2014 Jul;28(7):3090-102. doi: 10.1096/fj.13-248393. Epub 2014 Apr 1. FASEB J. 2014. PMID: 24692596
-
15-Epi-LXA4 and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation.Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):7971-7980. doi: 10.1073/pnas.1920193117. Epub 2020 Mar 23. Proc Natl Acad Sci U S A. 2020. PMID: 32205444 Free PMC article.
-
Regulation of the inflammatory response by vascular grafts modified with Aspirin-Triggered Resolvin D1 promotes blood vessel regeneration.Acta Biomater. 2019 Oct 1;97:360-373. doi: 10.1016/j.actbio.2019.07.037. Epub 2019 Jul 24. Acta Biomater. 2019. PMID: 31351251
-
Resolvin D1: A key endogenous inhibitor of neuroinflammation.Biofactors. 2022 Sep;48(5):1005-1026. doi: 10.1002/biof.1891. Epub 2022 Sep 29. Biofactors. 2022. PMID: 36176016 Review.
-
The role of Resolvin D1 in liver diseases.Prostaglandins Other Lipid Mediat. 2022 Jun;160:106634. doi: 10.1016/j.prostaglandins.2022.106634. Epub 2022 Mar 12. Prostaglandins Other Lipid Mediat. 2022. PMID: 35292355 Review.
Cited by
-
Lipids as mediators of cancer progression and metastasis.Nat Cancer. 2024 Jan;5(1):16-29. doi: 10.1038/s43018-023-00702-z. Epub 2024 Jan 25. Nat Cancer. 2024. PMID: 38273023 Review.
-
RNA Sequencing and Cell Models of Virus-Associated Cancer (Review).Sovrem Tekhnologii Med. 2022;14(1):64-80. doi: 10.17691/stm2022.14.1.07. Epub 2022 Jan 28. Sovrem Tekhnologii Med. 2022. PMID: 35992999 Free PMC article. Review.
-
Leveling Up the Controversial Role of Neutrophils in Cancer: When the Complexity Becomes Entangled.Cells. 2021 Sep 20;10(9):2486. doi: 10.3390/cells10092486. Cells. 2021. PMID: 34572138 Free PMC article. Review.
-
Neutrophils in cancer: dual roles through intercellular interactions.Oncogene. 2024 Apr;43(16):1163-1177. doi: 10.1038/s41388-024-03004-5. Epub 2024 Mar 12. Oncogene. 2024. PMID: 38472320 Review.
-
Eicosanoid regulation of debris-stimulated metastasis.Proc Natl Acad Sci U S A. 2021 Oct 12;118(41):e2107771118. doi: 10.1073/pnas.2107771118. Proc Natl Acad Sci U S A. 2021. PMID: 34607951 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

