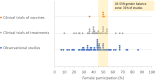
Inadequate reporting of COVID-19 clinical studies: a renewed rationale for the Sex and Gender Equity in Research (SAGER) guidelines
- PMID: 33846144
- PMCID: PMC8047552
- DOI: 10.1136/bmjgh-2021-004997
Inadequate reporting of COVID-19 clinical studies: a renewed rationale for the Sex and Gender Equity in Research (SAGER) guidelines
Keywords: COVID-19; clinical trial; epidemiology; health policy; systematic review.
Conflict of interest statement
Competing interests: SH is the founding chair of the Gender Policy Committee (GPC) of European Association of Science Editors (EASE), where she led the development and publication of the SAGER guidelines. She is the chair of and PVO is a member of the SAGER Guidelines Working Group of EASE GPC.
Figures
References
-
- Sudre C, Murray B, Varsavsky T. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms 2 collected by the Covid symptoms study app. medRxiv 2020. 10.1101/2020.10.19.20214494 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical