Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study
- PMID: 33846161
- PMCID: PMC9411904
- DOI: 10.1136/bjophthalmol-2020-318672
Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study
Abstract
Background: Brolucizumab has recently been approved in Europe as a novel treatment for patients with neovascular age-related macular degeneration (nAMD). We report on early experiences with real-world outcomes of switch to brolucizumab therapy in previously anti-vascular endothelial growth factor (anti-VEGF)-treated patients.
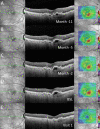
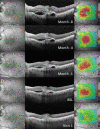
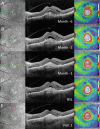
Methods: Patients with recalcitrant nAMD were switched to brolucizumab therapy. Functional and structural parameters 4 weeks after first brolucizumab injection were evaluated including best-corrected visual acuity (BCVA (logMAR)), foveal centre point (FCP (µm)), central subfield retinal thickness (CSRT (µm)) and macular volume (mm³).
Results: Sixty-three eyes of 57 patients with nAMD (52.6% females) with a mean (±SD) age of 79.5±6.7 years were included. Mean change of BCVA was 0.03±0.14 logMAR (p=0.115). Significant reductions were recorded for FCP with a mean (±SD) change of -66.81±72.63 µm, -66.76±60.71 µm for CSRT and -0.27±0.24 mm³ for macular volume (all p<0.001). Intraocular inflammation was observed in seven eyes of seven patients, including one case of retinal vasculitis.
Conclusions: The results of the SHIFT study indicate that switch to brolucizumab may represent a treatment option in patients with nAMD poorly responsive to other anti-VEGF agents. Further long-term analyses appear prudent to assess efficacy and safety of brolucizumab in a routine clinical setting.
Keywords: Brolucizumab; drugs; imaging; macula; neovascularisation.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Non-financial support from Heidelberg Engineering to ST, LB, RL, MS and FGH; from CenterVue to ST and FGH; from Optos to ST, LB, RL, MS and FGH; from Carl Zeiss Meditec to ST, LB, RF, FGH and MS. Grant and personal fees from Allergan, Novartis, Bayer and Heidelberg Engineering to ST and FGH; from Apellis, Carl Zeiss Meditec, Acucela, Genentech/Roche, Boehringer-Ingelheim, LIN Bioscience, Pixium, Kanghong, Oxurion, Grayburg Vision, Stealth BioTherapeutics, Geuder and Iveric Bio to FGH. JN: None declared.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
