Association between antihypertensive medications and risk of skin cancer in people older than 65 years: a population-based study
- PMID: 33846199
- PMCID: PMC8087333
- DOI: 10.1503/cmaj.201971
Association between antihypertensive medications and risk of skin cancer in people older than 65 years: a population-based study
Abstract
Background: The risk of skin cancer associated with antihypertensive medication use is unclear, although thiazides have been implicated in regulatory safety warnings. We aimed to assess whether use of thiazides and other antihypertensives is associated with increased rates of keratinocyte carcinoma and melanoma.
Methods: We conducted a population-based inception cohort study using linked administrative health data from Ontario, 1998-2017. We matched adults aged ≥ 66 years with a first prescription for an antihypertensive medication (thiazides, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, β-blockers) by age and sex to 2 unexposed adults who were prescribed a non-antihypertensive medication within 30 days of the index date. We evaluated each antihypertensive class in a separate cohort study. Our primary exposure was the cumulative dose within each class, standardized according to the World Health Organization's Defined Daily Dose. Outcomes were time to first keratinocyte carcinoma, advanced keratinocyte carcinoma and melanoma.
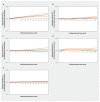
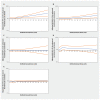
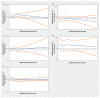
Results: The inception cohorts included a total of 302 634 adults prescribed an antihypertensive medication and 605 268 unexposed adults. Increasing thiazide exposure was associated with an increased rate of incident keratinocyte carcinoma (adjusted hazard ratios [HRs] per 1 Defined Annual Dose unit 1.08, 95% confidence interval [CI] 1.03-1.14), advanced keratinocyte carcinoma (adjusted HR 1.07, 95% CI 0.93-1.23) and melanoma (adjusted HR 1.34, 95% CI 1.01-1.78). We found no consistent evidence of association between other antihypertensive classes and keratinocyte carcinoma or melanoma.
Interpretation: Higher cumulative exposure to thiazides was associated with increased rates of incident skin cancer in people aged 66 years and older. Consideration of other antihypertensive treatments in patients at high risk of skin cancer may be warranted.
© 2021 Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Aaron Drucker reports receiving consulting fees from Sanofi, RTI Health Solutions, Eczema Society of Canada and Canadian Agency for Drugs and Technology in Health and honoraria from CME Outfitters. Dr. Drucker’s institutions have received educational grants from Sanofi (Women’s College Hospital ) and research grants from Sanofi and Regeneron (Brown University). Martin Weinstock has received consulting fees from AbbVie and Almirall. Husam Abdel-Qadir reports receiving personal fees from Amgen Canada and from the Canadian Vigour Centre, for time spent on an end-point adjudication committee for the THEMIS clinical trial. Dr. Abdel-Qadir also reports receiving grants from the Heart and Stroke Foundation of Canada, Canadian Institutes of Health Research, and the Canadian Cardiovascular Society. No other competing interests were declared.
Figures
Comment in
-
Association between thiazide diuretics and skin cancer: still nebulous.CMAJ. 2021 Jun 21;193(25):E963. doi: 10.1503/cmaj.78925. CMAJ. 2021. PMID: 34155049 Free PMC article. No abstract available.
-
The authors reply to: "Antihypertensives and skin cancer" and "Association between thiazide diuretics and skin cancer: still nebulous".CMAJ. 2021 Jun 21;193(25):E964. doi: 10.1503/cmaj.79145. CMAJ. 2021. PMID: 34155050 Free PMC article. No abstract available.
-
Consider patient risk factors for melanoma when prescribing antihypertensives.CMAJ. 2021 Jun 21;193(25):E996. doi: 10.1503/cmaj.78786. CMAJ. 2021. PMID: 34155058 Free PMC article. No abstract available.
Similar articles
-
Monotherapy With Major Antihypertensive Drug Classes and Risk of Hospital Admissions for Mood Disorders.Hypertension. 2016 Nov;68(5):1132-1138. doi: 10.1161/HYPERTENSIONAHA.116.08188. Epub 2016 Oct 10. Hypertension. 2016. PMID: 27733585 Free PMC article.
-
Antihypertensive drug prescribing and persistence among new elderly users: implications for persistence improvement interventions.Can J Cardiol. 2014 Jun;30(6):647-52. doi: 10.1016/j.cjca.2014.03.017. Epub 2014 Mar 18. Can J Cardiol. 2014. PMID: 24882536
-
Renin-Angiotensin-Aldosterone System-based Antihypertensive Agents and the Risk of Colorectal Cancer Among Medicare Beneficiaries.Epidemiology. 2019 Nov;30(6):867-875. doi: 10.1097/EDE.0000000000001065. Epidemiology. 2019. PMID: 31348009 Free PMC article.
-
50 years of thiazides: should thiazide diuretics be considered third-line hypertension treatment?Am J Ther. 2011 Nov;18(6):e244-54. doi: 10.1097/MJT.0b013e3181e90863. Am J Ther. 2011. PMID: 21436766 Review.
-
Clinical Pharmacology of Antihypertensive Therapy for the Treatment of Hypertension in CKD.Clin J Am Soc Nephrol. 2019 May 7;14(5):757-764. doi: 10.2215/CJN.04330418. Epub 2018 Nov 13. Clin J Am Soc Nephrol. 2019. PMID: 30425103 Free PMC article. Review.
Cited by
-
The phenomenon of phototoxicity and long-term risks of commonly prescribed and structurally diverse drugs.J Photochem Photobiol. 2024 Feb;19:100221. doi: 10.1016/j.jpap.2023.100221. Epub 2023 Dec 5. J Photochem Photobiol. 2024. PMID: 38389933 Free PMC article.
-
The Effect of Local Renin Angiotensin System in the Common Types of Cancer.Front Endocrinol (Lausanne). 2021 Sep 3;12:736361. doi: 10.3389/fendo.2021.736361. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34539580 Free PMC article. Review.
-
Neurobiology of Cancer: Introduction of New Drugs in the Treatment and Prevention of Cancer.Int J Mol Sci. 2021 Jun 6;22(11):6115. doi: 10.3390/ijms22116115. Int J Mol Sci. 2021. PMID: 34204103 Free PMC article. Review.
-
Photosensitizing Drugs and Risk of Skin Cancer in Women-A Prospective Population-Based Study.Photodermatol Photoimmunol Photomed. 2025 Mar;41(2):e70013. doi: 10.1111/phpp.70013. Photodermatol Photoimmunol Photomed. 2025. PMID: 40102189 Free PMC article.
-
Consider patient risk factors for melanoma when prescribing antihypertensives.CMAJ. 2021 Jun 21;193(25):E996. doi: 10.1503/cmaj.78786. CMAJ. 2021. PMID: 34155058 Free PMC article. No abstract available.
References
-
- Rogers HW, Weinstock MA, Feldman SR, et al. . Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol 2015;151:1081–6. - PubMed
-
- The economic burden of skin cancer in Canada: current and projected — final report. Toronto: Canadian Partnership Against Cancer; 2010.
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. - PubMed
-
- Glazer AM, Winkelmann RR, Farberg AS, et al. . Analysis of trends in US melanoma incidence and mortality. JAMA Dermatol 2017;153:225–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
