Racial and Ethnic Diversity in Studies Funded Under the Best Pharmaceuticals for Children Act
- PMID: 33846237
- PMCID: PMC9713833
- DOI: 10.1542/peds.2020-042903
Racial and Ethnic Diversity in Studies Funded Under the Best Pharmaceuticals for Children Act
Abstract
Background and objectives: The Best Pharmaceuticals for Children Act (BPCA) incentivizes the study of on-patent medicines in children and mandates that the National Institutes of Health sponsor research on off-patent drugs important to pediatric therapeutics. Failing to enroll cohorts that reflect the pediatric population at large restricts the generalizability of such studies. In this investigation, we evaluate racial and ethnic minority representation among participants enrolled in BPCA-sponsored studies.
Methods: Data were obtained for all participants enrolled in 33 federally funded studies of drugs and devices conducted from 2008 through June 2020. Observed racial and ethnic distributions were compared with expected distributions by sampling Census data at the same geographic frequency as in the studies. Racial and ethnic enrollment was examined by demography, geography, study type, study burden, and expected bias. Standard descriptive statistics, χ2, generalized linear models, and linear regression were applied.
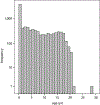
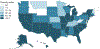
Results: A total of 10 918 participants (51% male, 6.6 ± 8.2 years) were enrolled across 46 US states and 4 countries. Studies ranged from treatment outcome reviews to randomized, placebo-controlled trials. Minority enrollment was comparable to, or higher than, expected (+0.1% to +2.6%) for all groups except Asian Americans (-3.7%, P < .001). American Indian and Alaskan Native and multiracial enrollment significantly increased over the evaluation period (P < .01). There were no significant differences in racial distribution as a function of age or sex, although differences were observed on the basis of geography, study type, and study burden.
Conclusions and relevance: This study revealed no evidence of racial and ethnic bias in enrollment for pediatric studies conducted with funding from BPCA, fulfilling the legislation's expectation to ensure adequate representation of all children.
Copyright © 2021 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Abdel-Rahman, Ms Delmore, and Drs Hornik, Paul, Sullivan, Wade, and Zimmerman serve as members of the steering committee for the Pediatric Trials Network, which receives Best Pharmaceuticals for Children Act funding from the Eunice Kennedy Shriver National Institute for Child Health and Human Development. Dr Benjamin serves as the PI of the Pediatric Trials Network. At the time of writing Dr Sharma was employed by the Best Pharmaceuticals for Children Act data coordinating center.
Figures
Comment in
-
Advocating for Minority Inclusion in Clinical Trials: A Call for Representation and Justice.Pediatrics. 2021 May;147(5):e2021049937. doi: 10.1542/peds.2021-049937. Epub 2021 Apr 12. Pediatrics. 2021. PMID: 33846236 No abstract available.
References
-
- Labeling for prescription drugs used in man; proposed format for prescription drug advertisements, 40 Fed. Reg. 15392 (April 7, 1975).
-
- Labeling and prescription drug advertising; content and format for labeling for human prescription drugs, 44 Fed. Reg. 37434 (June 26, 1979)
-
- Specific requirements on content and formal of labeling for human prescription drugs; proposed revision of “Pediatric use” subsection in the labeling. 57 Fed. Reg. 47423 (October 16, 1992)
-
- Specific requirements on content and formal of labeling for human prescription drugs; revision of “Pediatric use” subsection in the labeling. 59 Fed. Reg. 64240 (December 13, 1994)
-
- Forum on Drug Development, Institute of Medicine, National Academy of Sciences, Report of a Workshop on Drug Development and the Pediatric Population, Washington, DC, National Academy Press, 1991
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical