Simvastatin accelerated motoneurons death in SOD1G93A mice through inhibiting Rab7-mediated maturation of late autophagic vacuoles
- PMID: 33846297
- PMCID: PMC8041862
- DOI: 10.1038/s41419-021-03669-w
Simvastatin accelerated motoneurons death in SOD1G93A mice through inhibiting Rab7-mediated maturation of late autophagic vacuoles
Erratum in
-
Correction: Simvastatin accelerated motoneurons death in SOD1G93A mice through inhibiting Rab7-mediated maturation of late autophagic vacuoles.Cell Death Dis. 2025 Feb 27;16(1):139. doi: 10.1038/s41419-025-07349-x. Cell Death Dis. 2025. PMID: 40016232 Free PMC article. No abstract available.
Abstract
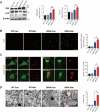
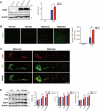
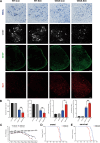
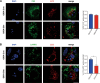
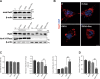
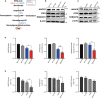
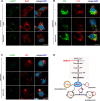
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease caused by motoneuron loss, for which there is currently no effective treatment. Statins, as inhibitors of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, are used as drugs for treatment for a variety of disease such as ischemic diseases, neurodegenerative diseases, cancer, and inflammation. However, our previous evidence has demonstrated that simvastatin leads to cytotoxicity in NSC34-hSOD1G93A cells by aggravating the impairment of autophagic flux, but the role of simvastatin in ALS model remains elusive. In present study, we reported that after simvastatin treatment, SOD1G93A mice showed early onset of the disease phenotype and shortened life span, with aggravated autophagic flux impairment and increased aggregation of SOD1 protein in spinal cord motoneurons (MNs) of SOD1G93A mice. In addition, simvastatin repressed the ability of Rab7 localization on the membrane by inhibiting isoprenoid synthesis, leading to impaired late stage of autophagic flux rather than initiation. This study suggested that simvastatin significantly worsened impairment of late autophagic flux, resulting in massive MNs death in spinal cord and accelerated disease progression of SOD1G93A mice. Together, these findings might imply a potential risk of clinic application of statins in ALS.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

