The E3 ligase MREL57 modulates microtubule stability and stomatal closure in response to ABA
- PMID: 33846350
- PMCID: PMC8041845
- DOI: 10.1038/s41467-021-22455-y
The E3 ligase MREL57 modulates microtubule stability and stomatal closure in response to ABA
Abstract
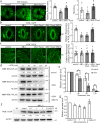
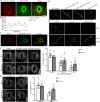
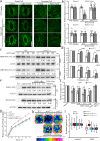
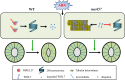
Regulation of stomatal movement is critical for plant adaptation to environmental stresses. The microtubule cytoskeleton undergoes disassembly, which is critical for stomatal closure in response to abscisic acid (ABA). However, the mechanism underlying this regulation largely remains unclear. Here we show that a ubiquitin-26S proteasome (UPS)-dependent pathway mediates microtubule disassembly and is required for ABA-induced stomatal closure. Moreover, we identify and characterize the ubiquitin E3 ligase MREL57 (MICROTUBULE-RELATED E3 LIGASE57) and the microtubule-stabilizing protein WDL7 (WAVE-DAMPENED2-LIKE7) in Arabidopsis and show that the MREL57-WDL7 module regulates microtubule disassembly to mediate stomatal closure in response to drought stress and ABA treatment. MREL57 interacts with, ubiquitinates and degrades WDL7, and this effect is clearly enhanced by ABA. ABA-induced stomatal closure and microtubule disassembly are significantly suppressed in mrel57 mutants, and these phenotypes can be restored when WDL7 expression is decreased. Our results unravel UPS-dependent mechanisms and the role of an MREL57-WDL7 module in microtubule disassembly and stomatal closure in response to drought stress and ABA.
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Guard cell regulation: pulling the strings behind the scenes.Trends Plant Sci. 2021 Nov;26(11):1093-1095. doi: 10.1016/j.tplants.2021.07.005. Epub 2021 Jul 21. Trends Plant Sci. 2021. PMID: 34303605
Similar articles
-
Arabidopsis RING E3 ubiquitin ligase JUL1 participates in ABA-mediated microtubule depolymerization, stomatal closure, and tolerance response to drought stress.Plant J. 2020 Jul;103(2):824-842. doi: 10.1111/tpj.14775. Epub 2020 May 2. Plant J. 2020. PMID: 32314432
-
The OPEN STOMATA1-SPIRAL1 module regulates microtubule stability during abscisic acid-induced stomatal closure in Arabidopsis.Plant Cell. 2023 Jan 2;35(1):260-278. doi: 10.1093/plcell/koac307. Plant Cell. 2023. PMID: 36255272 Free PMC article.
-
Arabidopsis RZFP34/CHYR1, a Ubiquitin E3 Ligase, Regulates Stomatal Movement and Drought Tolerance via SnRK2.6-Mediated Phosphorylation.Plant Cell. 2015 Nov;27(11):3228-44. doi: 10.1105/tpc.15.00321. Epub 2015 Oct 27. Plant Cell. 2015. PMID: 26508764 Free PMC article.
-
COP1 jointly modulates cytoskeletal processes and electrophysiological responses required for stomatal closure.Mol Plant. 2014 Sep;7(9):1441-1454. doi: 10.1093/mp/ssu065. Epub 2014 May 23. Mol Plant. 2014. PMID: 25151660 Free PMC article.
-
Role of Arabidopsis monomeric E3 ubiquitin ligases in the ABA signaling pathway.BMB Rep. 2025 Apr;58(4):147-157. doi: 10.5483/BMBRep.2024-0146. BMB Rep. 2025. PMID: 40058874 Free PMC article. Review.
Cited by
-
Enhanced photosynthetic efficiency by nitrogen-doped carbon dots via plastoquinone-involved electron transfer in apple.Hortic Res. 2024 Jan 12;11(3):uhae016. doi: 10.1093/hr/uhae016. eCollection 2024 Mar. Hortic Res. 2024. PMID: 38495032 Free PMC article.
-
Integration of light and ABA signaling pathways to combat drought stress in plants.Plant Cell Rep. 2023 May;42(5):829-841. doi: 10.1007/s00299-023-02999-7. Epub 2023 Mar 12. Plant Cell Rep. 2023. PMID: 36906730 Review.
-
Plant Stomata: An Unrealized Possibility in Plant Defense against Invading Pathogens and Stress Tolerance.Plants (Basel). 2023 Sep 25;12(19):3380. doi: 10.3390/plants12193380. Plants (Basel). 2023. PMID: 37836120 Free PMC article. Review.
-
F-box and WD repeat domain containing 7 inhibits the activation of hepatic stellate cells by degrading delta-like ligand 1 to block Notch signaling pathway.Open Med (Wars). 2023 Apr 17;18(1):20230634. doi: 10.1515/med-2023-0634. eCollection 2023. Open Med (Wars). 2023. PMID: 37082613 Free PMC article.
-
SPY Interacts With Tubulin and Regulates Abscisic Acid-Induced Stomatal Closure in Arabidopsis.Plant Direct. 2025 Apr 1;9(4):e70063. doi: 10.1002/pld3.70063. eCollection 2025 Apr. Plant Direct. 2025. PMID: 40170882 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

