Root NRT, NiR, AMT, GS, GOGAT and GDH expression levels reveal NO and ABA mediated drought tolerance in Brassica juncea L
- PMID: 33846385
- PMCID: PMC8041993
- DOI: 10.1038/s41598-021-86401-0
Root NRT, NiR, AMT, GS, GOGAT and GDH expression levels reveal NO and ABA mediated drought tolerance in Brassica juncea L
Abstract
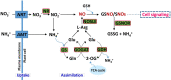
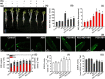
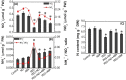
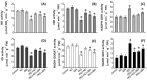
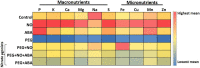
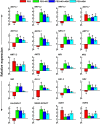
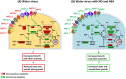
Little is known about the interactive effects of exogenous nitric oxide (NO) and abscisic acid (ABA) on nitrogen (N) metabolism and related changes at molecular and biochemical levels under drought stress. The present study highlights the independent and combined effect of NO and ABA (grouped as "nitrate agonists") on expression profiles of representative key genes known to be involved in N-uptake and assimilation, together with proline metabolism, N-NO metabolism enzyme's activity and nutrient content in polyethylene glycol (PEG) treated roots of Indian mustard (B. juncea cv. Varuna). Here we report that PEG mediated drought stress negatively inhibited growth performance, as manifested by reduced biomass (fresh and dry weight) production. Total N content and other nitrogenous compounds (NO3-, NO2-) were decreased; however, NH4+, NH4+/ NO3- ratio and total free amino acids content were increased. These results were positively correlated with the PEG induced changes in expression of genes and enzymes involved in N-uptake and assimilation. Also, PEG supply lowered the content of macro- and micro-nutrients but proline level and the activity of ∆1-pyrroline-5-carboxylate synthetase increased indicating increased oxidative stress. However, all these responses were reversed upon the exogenous application of nitrate agonists (PEG + NO, PEG + NO + ABA, and PEG + ABA) where NO containing nitrate agonist treatment i.e. PEG + NO was significantly more effective than PEG + ABA in alleviating drought stress. Further, increases in activities of L-arginine dependent NOS-like enzyme and S-nitrosoglutathione reductase were observed under nitrate agonist treatments. This indicates that the balanced endogenous change in NO and ABA levels together during synthesis and degradation of NO mitigated the oxidative stress in Indian mustard seedlings. Overall, our results reveal that NO independently or together with ABA may contribute to improved crop growth and productivity under drought stress.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Photosynthetic activity and RAPD profile of polyethylene glycol treated B. juncea L. under nitric oxide and abscisic acid application.J Biotechnol. 2020 Apr 10;313:29-38. doi: 10.1016/j.jbiotec.2020.03.004. Epub 2020 Mar 6. J Biotechnol. 2020. PMID: 32151644
-
Nitric oxide and abscisic acid protects against PEG-induced drought stress differentially in Brassica genotypes by combining the role of stress modulators, markers and antioxidants.Nitric Oxide. 2019 Aug 1;89:81-92. doi: 10.1016/j.niox.2019.05.005. Epub 2019 May 13. Nitric Oxide. 2019. PMID: 31096008
-
Ammonium uptake and metabolism alleviate PEG-induced water stress in rice seedlings.Plant Physiol Biochem. 2018 Nov;132:128-137. doi: 10.1016/j.plaphy.2018.08.041. Epub 2018 Aug 31. Plant Physiol Biochem. 2018. PMID: 30189416
-
Transcription Factors Interact with ABA through Gene Expression and Signaling Pathways to Mitigate Drought and Salinity Stress.Biomolecules. 2021 Aug 5;11(8):1159. doi: 10.3390/biom11081159. Biomolecules. 2021. PMID: 34439825 Free PMC article. Review.
-
Integration of nitrate and abscisic acid signaling in plants.J Exp Bot. 2024 Jun 7;75(11):3259-3268. doi: 10.1093/jxb/erae128. J Exp Bot. 2024. PMID: 38661493 Review.
Cited by
-
Abiotic stress tolerance in plants: a fascinating action of defense mechanisms.3 Biotech. 2023 Mar;13(3):102. doi: 10.1007/s13205-023-03519-w. Epub 2023 Feb 27. 3 Biotech. 2023. PMID: 36866326 Free PMC article. Review.
-
Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction.Biomolecules. 2023 Sep 25;13(10):1443. doi: 10.3390/biom13101443. Biomolecules. 2023. PMID: 37892125 Free PMC article. Review.
-
Polyethylene Glycol (PEG) Application Triggers Plant Dehydration but Does Not Accurately Simulate Drought.Plants (Basel). 2024 Dec 31;14(1):92. doi: 10.3390/plants14010092. Plants (Basel). 2024. PMID: 39795352 Free PMC article.
-
The impact of drought stress under different soil matrices on physiological characteristics of soybean seedlings.AoB Plants. 2025 Jul 16;17(4):plaf026. doi: 10.1093/aobpla/plaf026. eCollection 2025 Aug. AoB Plants. 2025. PMID: 40672978 Free PMC article.
-
Alleviation of drought stress in tomato by foliar application of seafood waste extract.Sci Rep. 2024 Dec 20;14(1):30572. doi: 10.1038/s41598-024-80798-0. Sci Rep. 2024. PMID: 39706919 Free PMC article.
References
-
- Panda DK, Wahr J. Spatiotemporal evolution of water storage changes in India from the updated GRACE-derived gravity records. Water Resour. Res. 2016;52:135–149. doi: 10.1002/2015WR017797. - DOI
-
- Food and Agricultural Organization . How to Feed the World in 2050. Food and Agricultural Organization; 2009. pp. 1–4.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

