Tick defensin γ-core reduces Fusarium graminearum growth and abrogates mycotoxins production with high efficiency
- PMID: 33846413
- PMCID: PMC8042122
- DOI: 10.1038/s41598-021-86904-w
Tick defensin γ-core reduces Fusarium graminearum growth and abrogates mycotoxins production with high efficiency
Abstract
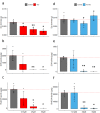
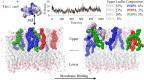
Fusarium graminearum is a major fungal pathogen affecting crops of worldwide importance. F. graminearum produces type B trichothecene mycotoxins (TCTB), which are not fully eliminated during food and feed processing. Therefore, the best way to minimize TCTB contamination is to develop prevention strategies. Herein we show that treatment with the reduced form of the γ-core of the tick defensin DefMT3, referred to as TickCore3 (TC3), decreases F. graminearum growth and abrogates TCTB production. The oxidized form of TC3 loses antifungal activity, but retains anti-mycotoxin activity. Molecular dynamics show that TC3 is recruited by specific membrane phospholipids in F. graminearum and that membrane binding of the oxidized form of TC3 is unstable. Capping each of the three cysteine residues of TC3 with methyl groups reduces its inhibitory efficacy. Substitutions of the positively-charged residues lysine (Lys) 6 or arginine 7 by threonine had the highest and the lesser impact, respectively, on the anti-mycotoxin activity of TC3. We conclude that the binding of linear TC3 to F. graminearum membrane phospholipids is required for the antifungal activity of the reduced peptide. Besides, Lys6 appears essential for the anti-mycotoxin activity of the reduced peptide. Our results provide foundation for developing novel and environment-friendly strategies for controlling F. graminearum.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Wilson W, Dahl B, Nganje W. Economic costs of Fusarium head blight, scab and deoxynivalenol. World Mycotoxin J. 2018;11:291–302. doi: 10.3920/WMJ2017.2204. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

