Abundance and size of hyaluronan in naked mole-rat tissues and plasma
- PMID: 33846452
- PMCID: PMC8041917
- DOI: 10.1038/s41598-021-86967-9
Abundance and size of hyaluronan in naked mole-rat tissues and plasma
Abstract
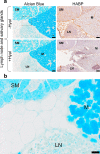
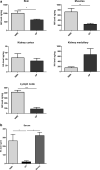
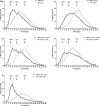
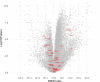
Large amounts of ultra-high molecular weight hyaluronan (HA) have been described as the main cause of cancer resistance in naked mole-rats (Heterocephalus glaber, NMR). Our work examined HA metabolism in these rodents more closely. HA was localized and quantified using HA binding proteins. Its molecular weight was determined using size exclusion chromatography and gel electrophoresis, HA family gene expression using RNAseq analysis, and hyaluronidase activity using zymography. Guinea pigs (Cavia porcellus) and mice (Mus musculus) were used as controls for some experiments. We found that HA localization was similar in NMR, guinea pig, and mouse tissues but NMR had larger amounts and higher molecular weight (maximum, around 2.5 MDa) of HA in serum and almost all tissues tested. We could not find ultra-high molecular weight HA (≥ 4 MDa) in NMR samples, in contrast to previous descriptions. Hyaluronidase-1 had lower expression and activity in NMR than mouse lymph nodes. RNAseq results showed that, among HA family genes, Tnfaip6 and hyaluronidase-3 (Hyal3) were systematically overexpressed in NMR tissues. In conclusion, NMR samples, contrary to expectations, do not harbor ultra-high molecular weight HA, although its amount and average molecular weight are higher in NMR than in guinea pig tissues and serum. Although hyaluronidase expression and activity are lower in NMR than mouse lymph nodes, this not sufficient to explain the presence of high molecular weight HA. A different activity of the NMR HA synthases remains possible. These characteristics, together with extremely high Hyal3 and Tnfaip6 expression, may provide the NMR with a bespoke, and perhaps protective, HA metabolism.
Conflict of interest statement
The authors have stated explicitely that there are no conflicts of interest. This work was partly supported by the Leibniz association competitive grants SAW-2012-FLI-2 and SAS-2016–2020-IZW-LFV. The study was carried out in compliance with the ARRIVE guidelines (
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

