Staphylococcus aureus vWF-binding protein triggers a strong interaction between clumping factor A and host vWF
- PMID: 33846500
- PMCID: PMC8041789
- DOI: 10.1038/s42003-021-01986-6
Staphylococcus aureus vWF-binding protein triggers a strong interaction between clumping factor A and host vWF
Abstract
The Staphylococcus aureus cell wall-anchored adhesin ClfA binds to the very large blood circulating protein, von Willebrand factor (vWF) via vWF-binding protein (vWbp), a secreted protein that does not bind the cell wall covalently. Here we perform force spectroscopy studies on living bacteria to unravel the molecular mechanism of this interaction. We discover that the presence of all three binding partners leads to very high binding forces (2000 pN), largely outperforming other known ternary complexes involving adhesins. Strikingly, our experiments indicate that a direct interaction involving features of the dock, lock and latch mechanism must occur between ClfA and vWF to sustain the extreme tensile strength of the ternary complex. Our results support a previously undescribed mechanism whereby vWbp activates a direct, ultra-strong interaction between ClfA and vWF. This intriguing interaction represents a potential target for therapeutic interventions, including synthetic peptides inhibiting the ultra-strong interactions between ClfA and its ligands.
Conflict of interest statement
The authors declare no competing interests.
Figures




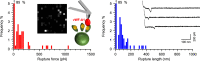

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

