Comparative performance of lung cancer risk models to define lung screening eligibility in the United Kingdom
- PMID: 33846525
- PMCID: PMC8184952
- DOI: 10.1038/s41416-021-01278-0
Comparative performance of lung cancer risk models to define lung screening eligibility in the United Kingdom
Erratum in
-
Correction: Comparative performance of lung cancer risk models to define lung screening eligibility in the United Kingdom.Br J Cancer. 2021 Jul;125(2):305. doi: 10.1038/s41416-021-01436-4. Br J Cancer. 2021. PMID: 34002041 Free PMC article. No abstract available.
Abstract
Background: The National Health Service England (NHS) classifies individuals as eligible for lung cancer screening using two risk prediction models, PLCOm2012 and Liverpool Lung Project-v2 (LLPv2). However, no study has compared the performance of lung cancer risk models in the UK.
Methods: We analysed current and former smokers aged 40-80 years in the UK Biobank (N = 217,199), EPIC-UK (N = 30,813), and Generations Study (N = 25,777). We quantified model calibration (ratio of expected to observed cases, E/O) and discrimination (AUC).
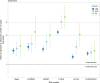
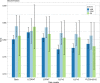
Results: Risk discrimination in UK Biobank was best for the Lung Cancer Death Risk Assessment Tool (LCDRAT, AUC = 0.82, 95% CI = 0.81-0.84), followed by the LCRAT (AUC = 0.81, 95% CI = 0.79-0.82) and the Bach model (AUC = 0.80, 95% CI = 0.79-0.81). Results were similar in EPIC-UK and the Generations Study. All models overestimated risk in all cohorts, with E/O in UK Biobank ranging from 1.20 for LLPv3 (95% CI = 1.14-1.27) to 2.16 for LLPv2 (95% CI = 2.05-2.28). Overestimation increased with area-level socioeconomic status. In the combined cohorts, USPSTF 2013 criteria classified 50.7% of future cases as screening eligible. The LCDRAT and LCRAT identified 60.9%, followed by PLCOm2012 (58.3%), Bach (58.0%), LLPv3 (56.6%), and LLPv2 (53.7%).
Conclusion: In UK cohorts, the ability of risk prediction models to classify future lung cancer cases as eligible for screening was best for LCDRAT/LCRAT, very good for PLCOm2012, and lowest for LLPv2. Our results highlight the importance of validating prediction tools in specific countries.
Conflict of interest statement
H.A.R., K.A., A.J.S., M.J.S., N.W., R.C.T., M.C., R.L., M.J.: None. P.A.J.C.: consultancy and share options, Everest Detection. D.R.B.: reimbursement for participation on advisory boards, AstraZeneca, and MSD.
Figures
References
-
- Cancer Research UK. Cancer Statistics for the UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics-f... (2020).
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. - PubMed
-
- de Koning, H. J., van der Aalst, C. M., de Jong, P. A., Scholten, E. T., Nackaerts, K., Heuvelmans, M. A. et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 382, 503–513 (2020). - PubMed
-
- Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014;160:330–338. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

