Control of IFN-I responses by the aminopeptidase IRAP in neonatal C57BL/6 alveolar macrophages during RSV infection
- PMID: 33846534
- PMCID: PMC8221999
- DOI: 10.1038/s41385-021-00402-w
Control of IFN-I responses by the aminopeptidase IRAP in neonatal C57BL/6 alveolar macrophages during RSV infection
Abstract
Respiratory Syncytial Virus (RSV) is the major cause of lower respiratory tract infection in infants, in whom, the sensing of RSV by innate immune receptors and its regulation are still poorly described. However, the severe bronchiolitis following RSV infection in neonates has been associated with a defect in type I interferons (IFN-I) production, a cytokine produced mainly by alveolar macrophages (AMs) upon RSV infection in adults. In the present study, neonatal C57BL/6 AMs mobilized very weakly the IFN-I pathway upon RSV infection in vitro and failed to restrain virus replication. However, IFN-I productions by neonatal AMs were substantially increased by the deletion of Insulin-Responsive AminoPeptidase (IRAP), a protein previously involved in the regulation of IFN-I production by dendritic cells. Moreover, neonatal IRAPKO AMs showed a higher expression of IFN-stimulated genes than their wild-type C57BL/6 counterpart. Interestingly, depletion of IRAP did not affect adult AM responses. Finally, we demonstrated that newborn IRAPKO mice infected with RSV had more IFN-I in their lungs and eliminated the virus more efficiently than WT neonates. Taken together, early-life susceptibility to RSV infection may be related to an original age-dependent suppressive function of IRAP on the IFN-I driven-antiviral responses in neonatal AMs.
Conflict of interest statement
The author declares no competing interests.
Figures


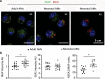

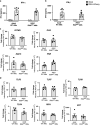



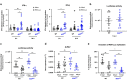
References
-
- Smyth RL, Openshaw PJ. Bronchiolitis. Lancet. 2006;368:312–322. - PubMed
-
- Janssen R, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J. Infect. Dis. 2007;196:826–834. - PubMed
-
- de Kleer IM, et al. Perinatal activation of the interleukin-33 pathway promotes Type 2 immunity in the developing lung. Immunity. 2016;45:1285–1298. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

