CARAMBA: a first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma
- PMID: 33846552
- PMCID: PMC8455317
- DOI: 10.1038/s41434-021-00254-w
CARAMBA: a first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma
Abstract
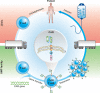
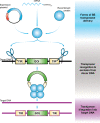
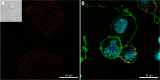
Clinical development of chimeric antigen receptor (CAR)-T-cell therapy has been enabled by advances in synthetic biology, genetic engineering, clinical-grade manufacturing, and complex logistics to distribute the drug product to treatment sites. A key ambition of the CARAMBA project is to provide clinical proof-of-concept for virus-free CAR gene transfer using advanced Sleeping Beauty (SB) transposon technology. SB transposition in CAR-T engineering is attractive due to the high rate of stable CAR gene transfer enabled by optimized hyperactive SB100X transposase and transposon combinations, encoded by mRNA and minicircle DNA, respectively, as preferred vector embodiments. This approach bears the potential to facilitate and expedite vector procurement, CAR-T manufacturing and distribution, and the promise to provide a safe, effective, and economically sustainable treatment. As an exemplary and novel target for SB-based CAR-T cells, the CARAMBA consortium has selected the SLAMF7 antigen in multiple myeloma. SLAMF7 CAR-T cells confer potent and consistent anti-myeloma activity in preclinical assays in vitro and in vivo. The CARAMBA clinical trial (Phase-I/IIA; EudraCT: 2019-001264-30) investigates the feasibility, safety, and anti-myeloma efficacy of autologous SLAMF7 CAR-T cells. CARAMBA is the first clinical trial with virus-free CAR-T cells in Europe, and the first clinical trial that uses advanced SB technology worldwide.
© 2021. The Author(s).
Conflict of interest statement
MH and ZI are co-inventors on patents relating to
Figures
References
-
- Narayanavari SA, Chilkunda SS, Ivics Z, Izsvak Z. Sleeping Beauty transposition: from biology to applications. Sleeping Beauty. 2017;52:18–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

