Minor intron retention drives clonal hematopoietic disorders and diverse cancer predisposition
- PMID: 33846634
- PMCID: PMC8177065
- DOI: 10.1038/s41588-021-00828-9
Minor intron retention drives clonal hematopoietic disorders and diverse cancer predisposition
Abstract
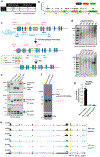
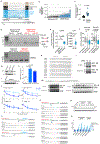
Most eukaryotes harbor two distinct pre-mRNA splicing machineries: the major spliceosome, which removes >99% of introns, and the minor spliceosome, which removes rare, evolutionarily conserved introns. Although hypothesized to serve important regulatory functions, physiologic roles of the minor spliceosome are not well understood. For example, the minor spliceosome component ZRSR2 is subject to recurrent, leukemia-associated mutations, yet functional connections among minor introns, hematopoiesis and cancers are unclear. Here, we identify that impaired minor intron excision via ZRSR2 loss enhances hematopoietic stem cell self-renewal. CRISPR screens mimicking nonsense-mediated decay of minor intron-containing mRNA species converged on LZTR1, a regulator of RAS-related GTPases. LZTR1 minor intron retention was also discovered in the RASopathy Noonan syndrome, due to intronic mutations disrupting splicing and diverse solid tumors. These data uncover minor intron recognition as a regulator of hematopoiesis, noncoding mutations within minor introns as potential cancer drivers and links among ZRSR2 mutations, LZTR1 regulation and leukemias.
Conflict of interest statement
COMPETING INTERESTS
O.A.-W. has served as a consultant for H3B Biomedicine, Foundation Medicine Inc, Merck, Prelude Therapeutics, and Janssen, and is on the Scientific Advisory Board of Envisagenics Inc., AIChemy, and Pfizer Boulder; O.A.-W. has received prior research funding from H3B Biomedicine and LOXO Oncology unrelated to the current manuscript. The remaining authors declare no competing interests.
Figures






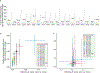






References
-
- Tarn WY & Steitz JA A novel spliceosome containing U11, U12, and U5 snRNPs excises a minor class (AT-AC) intron in vitro. Cell 84, 801–11 (1996). - PubMed
-
- Hall SL & Padgett RA Conserved sequences in a class of rare eukaryotic nuclear introns with non-consensus splice sites. J Mol Biol 239, 357–65 (1994). - PubMed
-
- Tarn WY & Steitz JA Highly diverged U4 and U6 small nuclear RNAs required for splicing rare AT-AC introns. Science 273, 1824–32 (1996). - PubMed
-
- Hall SL & Padgett RA Requirement of U12 snRNA for in Vivo Splicing of a Minor Class of Eukaryotic Nuclear Pre-mRNA Introns. Science 271, 1716–1718 (1996). - PubMed
-
- Burge CB, Padgett RA & Sharp PA Evolutionary fates and origins of U12-type introns. Mol Cell 2, 773–85 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

