Vascular smooth muscle-derived Trpv1+ progenitors are a source of cold-induced thermogenic adipocytes
- PMID: 33846638
- PMCID: PMC8076094
- DOI: 10.1038/s42255-021-00373-z
Vascular smooth muscle-derived Trpv1+ progenitors are a source of cold-induced thermogenic adipocytes
Abstract
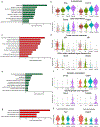
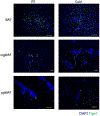
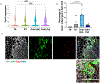
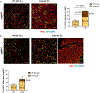
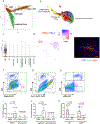
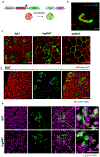
Brown adipose tissue (BAT) and beige fat function in energy expenditure in part due to their role in thermoregulation, making these tissues attractive targets for treating obesity and metabolic disorders. While prolonged cold exposure promotes de novo recruitment of brown adipocytes, the exact sources of cold-induced thermogenic adipocytes are not completely understood. Here, we identify transient receptor potential cation channel subfamily V member 1 (Trpv1)+ vascular smooth muscle (VSM) cells as previously unidentified thermogenic adipocyte progenitors. Single-cell RNA sequencing analysis of interscapular brown adipose depots reveals, in addition to the previously known platelet-derived growth factor receptor (Pdgfr)α-expressing mesenchymal progenitors, a population of VSM-derived adipocyte progenitor cells (VSM-APC) expressing the temperature-sensitive cation channel Trpv1. We demonstrate that cold exposure induces the proliferation of Trpv1+ VSM-APCs and enahnces their differentiation to highly thermogenic adipocytes. Together, these findings illustrate the landscape of the thermogenic adipose niche at single-cell resolution and identify a new cellular origin for the development of brown and beige adipocytes.
Figures





Comment in
-
Identification of thermogenic adipocyte lineages.Nat Rev Endocrinol. 2021 Jun;17(6):319. doi: 10.1038/s41574-021-00497-y. Nat Rev Endocrinol. 2021. PMID: 33907309 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

