Real-World Experience of Bamlanivimab for Coronavirus Disease 2019 (COVID-19): A Case-Control Study
- PMID: 33846730
- PMCID: PMC8083260
- DOI: 10.1093/cid/ciab305
Real-World Experience of Bamlanivimab for Coronavirus Disease 2019 (COVID-19): A Case-Control Study
Abstract
Background: Coronavirus disease 2019 (COVID-19) has strained healthcare systems with patient hospitalizations and deaths. Anti-spike monoclonal antibodies, including bamlanivimab, have demonstrated reduction in hospitalization rates in clinical trials, yet real-world evidence is lacking.
Methods: We conducted a retrospective case-control study across a single healthcare system of nonhospitalized patients, age 18 years or older, with documented positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing, risk factors for severe COVID-19, and referrals for bamlanivimab via emergency use authorization. Cases were defined as patients who received bamlanivimab; contemporary controls had a referral order placed but did not receive bamlanivimab. The primary outcome was 30-day hospitalization rate from initial positive SARS-CoV-2 polymerase chain reaction (PCR). Descriptive statistics, including χ 2 and Mann-Whitney U test, were performed. Multivariable logistic regression was used for adjusted analysis to evaluate independent associations with 30-day hospitalization.
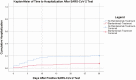
Results: Between 30 November 2020 and 19 January 2021, 218 patients received bamlanivimab (cases), and 185 were referred but did not receive drug (controls). Thirty-day hospitalization rate was significantly lower among patients who received bamlanivimab (7.3% vs 20.0%, risk ratio [RR] 0.37, 95% confidence interval [CI]: .21-.64, P < .001), and the number needed to treat was 8. On logistic regression, odds of hospitalization were increased in patients not receiving bamlanivimab and with a higher number of pre-specified comorbidities (odds ratio [OR] 4.19 ,95% CI: 1.31-2.16, P < .001; OR 1.68, 95% CI: 2.12-8.30, P < .001, respectively).
Conclusions: Ambulatory patients with COVID-19 who received bamlanivimab had a lower 30-day hospitalization than control patients in real-world experience. We identified receipt of bamlanivimab and fewer comorbidities as protective factors against hospitalization.Bamlanivimab's role in preventing hospitalization associated with coronavirus disease 2019 (COVID-19) remains unclear. In a real-world, retrospective study of 403 high-risk, ambulatory patients with COVID-19, receipt of bamlanivimab compared to no monoclonal antibody therapy was associated with lower 30-day hospitalization.
Keywords: COVID-19; SARS-CoV-2; bamlanivimab; monoclonal antibody.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Bamlanivimab Treatment Leads to Rapid Selection of Immune Escape Variant Carrying the E484K Mutation in a B.1.1.7-Infected and Immunosuppressed Patient.Clin Infect Dis. 2021 Dec 6;73(11):2144-2145. doi: 10.1093/cid/ciab392. Clin Infect Dis. 2021. PMID: 34009286 No abstract available.
-
Reply to Lapadula et al.Clin Infect Dis. 2022 Jan 29;74(2):363-364. doi: 10.1093/cid/ciab468. Clin Infect Dis. 2022. PMID: 34015090 Free PMC article. No abstract available.
-
Beware of Biases in Observational Studies on Anti-Spike Monoclonal Antibodies.Clin Infect Dis. 2022 Jan 29;74(2):363. doi: 10.1093/cid/ciab467. Clin Infect Dis. 2022. PMID: 34015133 Free PMC article. No abstract available.
-
Early Monoclonal Antibody Administration Can Reduce Both Hospitalizations and Mortality in High-Risk Outpatients With Coronavirus Disease 2019 (COVID-19).Clin Infect Dis. 2022 Mar 1;74(4):752-753. doi: 10.1093/cid/ciab522. Clin Infect Dis. 2022. PMID: 34091664 Free PMC article. No abstract available.
References
-
- WHO coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 28 February.
-
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins (JHU). Available at: https://coronavirus.jhu.edu/map.html. Accessed 28 February.
-
- FDA. approves first treatment for COVID-19.https://www.fda.gov/news-events/press-announcements/fda-approves-first-t.... Published October 22, 2020. Accessed 20 May 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous