Astragaloside IV alleviates liver injury in type 2 diabetes due to promotion of AMPK/mTOR‑mediated autophagy
- PMID: 33846768
- PMCID: PMC8060804
- DOI: 10.3892/mmr.2021.12076
Astragaloside IV alleviates liver injury in type 2 diabetes due to promotion of AMPK/mTOR‑mediated autophagy
Abstract
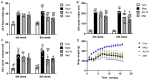
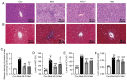
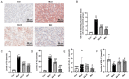
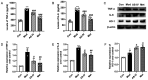
Diabetic liver injury is a serious complication of type 2 diabetes mellitus (T2DM), which is often irreversible in the later stage, and affects the quality of life. Autophagy serves an important role in the occurrence and development of diabetic liver injury. For example, it can improve insulin resistance (IR), dyslipidaemia, oxidative stress and inflammation. Astragaloside IV (AS‑IV) is a natural saponin isolated from the plant Astragalus membranaceus, which has comprehensive pharmacological effects, such as anti‑oxidation, anti‑inflammation and anti‑apoptosis properties, as well as can enhance immunity. However, whether AS‑IV can alleviate diabetic liver injury in T2DM and its underlying mechanisms remain unknown. The present study used high‑fat diets combined with low‑dose streptozotocin to induce a diabetic liver injury model in T2DM rats to investigate whether AS‑IV could alleviate diabetic liver injury and to identify its underlying mechanisms. The results demonstrated that AS‑IV treatment could restore changes in food intake, water intake, urine volume and body weight, as well as improve liver function and glucose homeostasis in T2DM rats. Moreover, AS‑IV treatment promoted suppressed autophagy in the liver of T2DM rats and improved IR, dyslipidaemia, oxidative stress and inflammation. In addition, AS‑IV activated adenosine monophosphate‑activated protein kinase (AMPK), which inhibited mTOR. Taken together, the present study suggested that AS‑IV alleviated diabetic liver injury in T2DM rats, and its mechanism may be associated with the promotion of AMPK/mTOR‑mediated autophagy, which further improved IR, dyslipidaemia, oxidative stress and inflammation. Thus, the regulation of autophagy may be an effective strategy to treat diabetic liver injury in T2DM.
Keywords: AS‑IV; diabetic liver injury; autophagy; AMPK; mTOR.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Tan SY, Mei Wong JL, Sim YJ, Wong SS, Mohamed Elhassan SA, Tan SH, Ling Lim GP, Rong Tay NW, Annan NC, Bhattamisra SK, Candasamy M. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab Syndr. 2019;13:364–372. doi: 10.1016/j.dsx.2018.10.008. - DOI - PubMed
-
- Shima T, Uto H, Ueki K, Takamura T, Kohgo Y, Kawata S, Yasui K, Park H, Nakamura N, Nakatou T, et al. Clinicopathological features of liver injury in patients with type 2 diabetes mellitus and comparative study of histologically proven nonalcoholic fatty liver diseases with or without type 2 diabetes mellitus. J Gastroenterol. 2013;48:515–525. doi: 10.1007/s00535-012-0653-5. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

