A blocking peptide stabilizes lysophosphatidic acid receptor 1 and promotes lysophosphatidic acid-induced cellular responses
- PMID: 33847006
- PMCID: PMC8934197
- DOI: 10.1002/jcb.29919
A blocking peptide stabilizes lysophosphatidic acid receptor 1 and promotes lysophosphatidic acid-induced cellular responses
Abstract
G protein-coupled receptors regulate a variety of cellular responses and have been considered as therapeutic targets for human diseases. Lysophosphatidic acid receptor 1 (LPA1) is a receptor for bioactive lysophospholipid, LPA. LPA/LPA1-mediated signaling contributes to inflammatory and fibrotic responses in lung diseases; thus understanding regulation of LPA1 stability is important for modulating LPA/LPA1 signaling. Our previous study has shown that LPA1 is degraded in the Nedd4 like (Nedd4L) E3 ubiquitin ligase-mediated ubiquitin-proteasome system. In the current study, we attempt to identify a peptide that stabilizes LPA1 through disrupting LPA1 association with Nedd4L. LPA treatment induces both endogenous and overexpressed LPA1 degradation, which is attenuated by a proteasome inhibitor, suggesting that LPA1 is degraded in the proteasome. LPA increases phosphorylation of extracellular signal-regulated kinase 1/2 (Erk1/2) and I-κB kinase in lung epithelial cells, and this effect is promoted by overexpression of a peptide (P1) that mimics C-terminal of LPA1. P1, not a control peptide, attenuates LPA-induced LPA1 ubiquitination and degradation, suggesting that P1 stabilizes LPA1. Further, P1 diminishes Nedd4L-mediated degradation of LPA1 and Nedd4L/LPA1 association. In addition to increasing LPA1 signaling, P1 enhances LPA-induced cell migration and gene expression of Elafin, matrix metallopeptidase 1, and serpin family B member 2 in lung epithelial cells. These data suggest that disruption of LPA1 interaction with Nedd4L by P1 increases LPA1 stability and LPA/LPA1 signaling.
Keywords: E3 ubiquitin ligase; GPCR; LPA1; blocking peptide; degradation; ubiquitination.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Figures
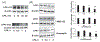
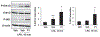
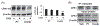




Similar articles
-
Molecular Regulation of Lysophosphatidic Acid Receptor 1 Maturation and Desensitization.Cell Biochem Biophys. 2021 Sep;79(3):477-483. doi: 10.1007/s12013-021-00999-6. Epub 2021 May 25. Cell Biochem Biophys. 2021. PMID: 34032994 Free PMC article. Review.
-
Destabilization of Lysophosphatidic Acid Receptor 1 Reduces Cytokine Release and Protects Against Lung Injury.EBioMedicine. 2016 Aug;10:195-203. doi: 10.1016/j.ebiom.2016.07.020. Epub 2016 Jul 18. EBioMedicine. 2016. PMID: 27448760 Free PMC article.
-
Cross-talk between lysophosphatidic acid receptor 1 and tropomyosin receptor kinase A promotes lung epithelial cell migration.Biochim Biophys Acta. 2016 Feb;1863(2):229-35. doi: 10.1016/j.bbamcr.2015.11.012. Epub 2015 Nov 17. Biochim Biophys Acta. 2016. PMID: 26597701 Free PMC article.
-
LPA receptor1 antagonists as anticancer agents suppress human lung tumours.Eur J Pharmacol. 2020 Feb 5;868:172886. doi: 10.1016/j.ejphar.2019.172886. Epub 2019 Dec 19. Eur J Pharmacol. 2020. PMID: 31866407
-
Lysophosphatidic acid (LPA) receptor-mediated signaling and cellular responses to anticancer drugs and radiation of cancer cells.Adv Biol Regul. 2024 May;92:101029. doi: 10.1016/j.jbior.2024.101029. Epub 2024 Feb 14. Adv Biol Regul. 2024. PMID: 38377635 Review.
Cited by
-
Unraveling the Role of Autotaxin and Lysophosphatidic Acid in Alzheimer's Disease: From Molecular Mechanisms to Therapeutic Potential.Int J Mol Sci. 2025 Jul 23;26(15):7068. doi: 10.3390/ijms26157068. Int J Mol Sci. 2025. PMID: 40806201 Free PMC article. Review.
-
Molecular Regulation of Lysophosphatidic Acid Receptor 1 Maturation and Desensitization.Cell Biochem Biophys. 2021 Sep;79(3):477-483. doi: 10.1007/s12013-021-00999-6. Epub 2021 May 25. Cell Biochem Biophys. 2021. PMID: 34032994 Free PMC article. Review.
-
Targeting GPCRs to treat cardiac fibrosis.Front Cardiovasc Med. 2022 Oct 6;9:1011176. doi: 10.3389/fcvm.2022.1011176. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36277752 Free PMC article. Review.
-
Targeting cardiac fibrosis with Chimeric Antigen Receptor-Engineered Cells.Mol Cell Biochem. 2025 Apr;480(4):2103-2116. doi: 10.1007/s11010-024-05134-6. Epub 2024 Oct 26. Mol Cell Biochem. 2025. PMID: 39460827 Review.
-
Extracellular Lipids in the Lung and Their Role in Pulmonary Fibrosis.Cells. 2022 Apr 3;11(7):1209. doi: 10.3390/cells11071209. Cells. 2022. PMID: 35406772 Free PMC article. Review.
References
-
- Johnson EN, Druey KM. Heterotrimeric G protein signaling: role in asthma and allergic inflammation. J Allergy Clin Immunol. 2002;109:592–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

