A Multicenter, Double-Blind, Randomized, Placebo-Controlled Phase 2b Trial of Cytisinicline in Adult Smokers (The ORCA-1 Trial)
- PMID: 33847362
- PMCID: PMC8403245
- DOI: 10.1093/ntr/ntab073
A Multicenter, Double-Blind, Randomized, Placebo-Controlled Phase 2b Trial of Cytisinicline in Adult Smokers (The ORCA-1 Trial)
Abstract
Introduction: Cytisinicline (known as cytisine), a nicotinic acetylcholine receptor partial agonist, is a smoking cessation aid currently marketed in Central and Eastern Europe using a 1.5-mg/tablet 25-day downward titration schedule. No prior studies have evaluated other doses or administration schedules. This study evaluated the effects of a higher dosage and simplified dosing schedule on drug efficacy and tolerability.
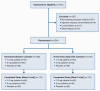
Methods: ORCA-1 was a double-blind, randomized, placebo-controlled clinical trial that provided cytisinicline or placebo tablets plus behavioral support for 25 days. Adult smokers (>10 cigarettes daily) committed to quitting smoking were randomized to compare 2 cytisinicline doses (1.5 mg and 3 mg) versus placebo, and 2 administration schedules [downward titration versus 3 times daily (TID)]. Primary outcome was a reduction in expected cigarettes smoked at end of treatment; secondary outcomes were biochemically confirmed 7-day abstinence at Week 4 and continuous abstinence from Weeks 5 to 8.
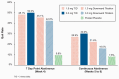
Results: Among 254 participants, those in cytisinicline arms (regardless of dose or schedule) had greater reductions in cigarettes smoked versus placebo, with differences observed in 3 cytisinicline arms statistically significant versus placebo. All cytisinicline arms had statistically significantly higher abstinence rates at Week 4 versus placebo. Both cytisinicline arms using TID schedules had statistically significantly higher continuous abstinence rates from Weeks 5 to 8 compared with placebo. Participants in the cytisinicline 3-mg TID arm had the highest abstinence rate. There were no safety concerns with either 1.5-mg or 3-mg cytisinicline.
Conclusion: Based on simpler dose scheduling, excellent tolerability, and best-continued abstinence rate, cytisinicline 3-mg TID was selected for future Phase 3 studies.
Implications: Although the 1.5-mg 25-day titration schedule has been marketed in Central and Eastern Europe for decades, this study explored using a higher dosage and a simplified dosing schedule for impact on cytisinicline efficacy and tolerability. Based on these results, a Phase 3 program was initiated using cytisinicline 3-mg tablets on a TID schedule for potential market approval in the United States.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco.
Figures
References
-
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2019: Monitoring Tobacco Use and Prevention Policies. Geneva: World Health Organization; 2019.
-
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. - PubMed
-
- Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: An alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–3477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources