Quantifying noxious-evoked baseline sensitivity in neonates to optimise analgesic trials
- PMID: 33847561
- PMCID: PMC8087440
- DOI: 10.7554/eLife.65266
Quantifying noxious-evoked baseline sensitivity in neonates to optimise analgesic trials
Abstract
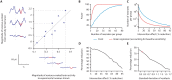
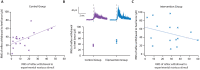
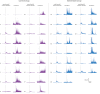
Despite the high burden of pain experienced by hospitalised neonates, there are few analgesics with proven efficacy. Testing analgesics in neonates is experimentally and ethically challenging and minimising the number of neonates required to demonstrate efficacy is essential. EEG (electroencephalography)-derived measures of noxious-evoked brain activity can be used to assess analgesic efficacy; however, as variability exists in neonate's responses to painful procedures, large sample sizes are often required. Here, we present an experimental paradigm to account for individual differences in noxious-evoked baseline sensitivity which can be used to improve the design of analgesic trials in neonates. The paradigm is developed and tested across four observational studies using clinical, experimental, and simulated data (92 neonates). We provide evidence of the efficacy of gentle brushing and paracetamol, substantiating the need for randomised controlled trials of these interventions. This work provides an important step towards safe, cost-effective clinical trials of analgesics in neonates.
Keywords: analgesic clinical trials; baseline sensitivity; human; medicine; neonates; neuroscience.
Plain language summary
Hospitalized newborns often undergo medical procedures, like blood tests, without pain relief. This can cause the baby to experience short-term distress that may have negative consequences later in life. However, testing the effects of pain relief in newborns is challenging because, unlike adults, they cannot report how much pain they are experiencing. One way to overcome this is to record the brain activity of newborns during a painful procedure and to see how these signals are modified following pain relief. Randomized controlled trials are the gold standard for these kinds of medical assessments, but require a high number of participants to account for individual differences in how babies respond to pain. Finding ways to reduce the size of pain control studies could lead to faster development of pain relief methods. Here, Cobo, Hartley et al. demonstrate a way to reduce the number of newborns needed to test potential pain-relieving interventions. In the experiments, the brain activity of nine babies was measured after a gentle poke and after a painful clinically required procedure. Cobo, Hartley et al. found that the babies’ response to the gentle poke correlated with their response to pain. Further data analysis revealed that this information can be used to predict the variability in pain experienced by different newborns, reducing the number of participants needed for pain relief trials. Next, Cobo, Hartley et al. used this new approach in two pilot tests. One showed that gently stroking an infant’s leg before blood is drawn from their heel reduced their brains’ response to pain. The second showed that giving a baby the painkiller paracetamol lessened the brain’s response to immunisation. The new approach identified by Cobo, Hartley et al. may enable smaller studies that can more quickly identify ways to reduce pain in babies. Furthermore, this work suggests that gentle brushing and paracetamol could provide pain relief for newborns undergoing hospital acute procedures. However, more formal clinical trials are needed to test the effectiveness of these two strategies.
© 2021, Cobo et al.
Conflict of interest statement
MC, CH, DG, FA, Mv, GS, LB, ED, MB, RE, GG, AH, RR, EA, FM, RS No competing interests declared
Figures





References
-
- Allegaert K. Clinical studies in infants (pediatric pharmacology) In: Hock F, Gralinski M, editors. Drug Discovery and Evaluation: Methods in Clinical Pharmacology. Springer; 2017. pp. 1–16. - DOI
-
- Anand KJS, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. The Lancet. 2004;363:1673–1682. doi: 10.1016/S0140-6736(04)16251-X. - DOI - PubMed
-
- Ancora G, Lago P, Garetti E, Pirelli A, Merazzi D, Mastrocola M, Pierantoni L, Faldella G. Efficacy and safety of continuous infusion of fentanyl for pain control in preterm newborns on mechanical ventilation. The Journal of Pediatrics. 2013;163:645–651. doi: 10.1016/j.jpeds.2013.02.039. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

