SKI activates the Hippo pathway via LIMD1 to inhibit cardiac fibroblast activation
- PMID: 33847835
- PMCID: PMC8043893
- DOI: 10.1007/s00395-021-00865-9
SKI activates the Hippo pathway via LIMD1 to inhibit cardiac fibroblast activation
Abstract
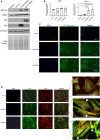
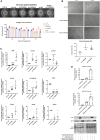
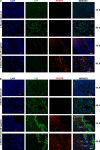
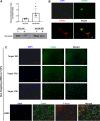
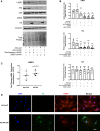
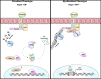
We have previously shown that overexpression of SKI, an endogenous TGF-β1 repressor, deactivates the pro-fibrotic myofibroblast phenotype in the heart. We now show that SKI also functions independently of SMAD/TGF-β signaling, by activating the Hippo tumor-suppressor pathway and inhibiting the Transcriptional co-Activator with PDZ-binding motif (TAZ or WWTR1). The mechanism(s) by which SKI targets TAZ to inhibit cardiac fibroblast activation and fibrogenesis remain undefined. A rat model of post-myocardial infarction was used to examine the expression of TAZ during acute fibrogenesis and chronic heart failure. Results were then corroborated with primary rat cardiac fibroblast cell culture performed both on plastic and on inert elastic substrates, along with the use of siRNA and adenoviral expression vectors for active forms of SKI, YAP, and TAZ. Gene expression was examined by qPCR and luciferase assays, while protein expression was examined by immunoblotting and fluorescence microscopy. Cell phenotype was further assessed by functional assays. Finally, to elucidate SKI's effects on Hippo signaling, the SKI and TAZ interactomes were captured in human cardiac fibroblasts using BioID2 and mass spectrometry. Potential interactors were investigated in vitro to reveal novel mechanisms of action for SKI. In vitro assays on elastic substrates revealed the ability of TAZ to overcome environmental stimuli and induce the activation of hypersynthetic cardiac myofibroblasts. Further cell-based assays demonstrated that SKI causes specific proteasomal degradation of TAZ, but not YAP, and shifts actin cytoskeleton dynamics to inhibit myofibroblast activation. These findings were supported by identifying the bi-phasic expression of TAZ in vivo during post-MI remodeling and fibrosis. BioID2-based interactomics in human cardiac fibroblasts suggest that SKI interacts with actin-modifying proteins and with LIM Domain-containing protein 1 (LIMD1), a negative regulator of Hippo signaling. Furthermore, we found that LATS2 interacts with TAZ, whereas LATS1 does not, and that LATS2 knockdown prevented TAZ downregulation with SKI overexpression. Our findings indicate that SKI's capacity to regulate cardiac fibroblast activation is mediated, in part, by Hippo signaling. We postulate that the interaction between SKI and TAZ in cardiac fibroblasts is arbitrated by LIMD1, an important intermediary in focal adhesion-associated signaling pathways. This study contributes to the understanding of the unique physiology of cardiac fibroblasts, and of the relationship between SKI expression and cell phenotype.
Keywords: Cardiac fibrosis; Extracellular matrix; Fibroblast; Hippo signaling; SKI; TAZ.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Bai SW, Herrera-Abreu MT, Rohn JL, Racine V, Tajadura V, Suryavanshi N, Bechtel S, Wiemann S, Baum B, Ridley AJ. Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biol. 2011;9:54. doi: 10.1186/1741-7007-9-54. - DOI - PMC - PubMed
-
- Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang YY, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Black SM, Fratz S, Fineman JR, Vargas SO, Haley KJ, Waxman AB, Chau BN, Fredenburgh LE, Chan SY. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. - DOI - PMC - PubMed
-
- Bhandary B, Meng Q, James J, Osinska H, Gulick J, Valiente-Alandi I, Sargent MA, Bhuiyan MS, Blaxall BC, Molkentin JD, Robbins J. Cardiac fibrosis in proteotoxic cardiac disease is dependent upon myofibroblast TGF -beta signaling. JAHA. 2018;7:e010013. doi: 10.1161/JAHA.118.010013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

