The Activity of Crizotinib in Chemo-Refractory MET-Amplified Esophageal and Gastric Adenocarcinomas: Results from the AcSé-Crizotinib Program
- PMID: 33847874
- PMCID: PMC8105218
- DOI: 10.1007/s11523-021-00811-8
The Activity of Crizotinib in Chemo-Refractory MET-Amplified Esophageal and Gastric Adenocarcinomas: Results from the AcSé-Crizotinib Program
Abstract
Background: The AcSé-crizotinib program provides extensive screening of crizotinib-targeted genomic alteration in several malignancies. We here report the results in patients with esogastric MET-amplified adenocarcinomas.
Objective: The objective of the study was to evaluate the efficacy and tolerability of crizotinib in patients with pretreated esogastric MET-amplified adenocarcinoma who have no alternative treatment options.
Patients and methods: MET expression was evaluated by fluorescence in situ hybridization in tumor samples with immunohistochemistry scores ≥ 2+. Patients with chemo-refractory tumors showing ≥ 6 MET copies were eligible for crizotinib 250 mg twice daily. The primary efficacy outcome was the objective response rate after two cycles of crizotinib.
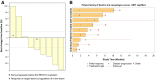
Results: MET was prospectively analyzed in 570 esogastric adenocarcinomas. Amplifications were found in 35/570 adenocarcinomas (29/523 gastric and 6/47 esophageal). Nine patients were treated with crizotinib. The objective response rate after two cycles was 33.3% (95% CI 7.5-70), the best overall response rate was 55.6% (95% CI 21.2-86.3), with median progression-free survival of 3.2 months (95% CI 1.0-5.4), and overall survival of 8.1 months (95% CI 1.7-24.6). Safety was consistent with that previously reported for crizotinib.
Conclusions: Large-scale screening for MET-amplified esogastric adenocarcinomas is feasible. MET amplification was observed in 5.5% of gastric and 12.8% of esophageal adenocarcinomas. Crizotinib shows encouraging results in selected patients. Thus, c-MET inhibition for MET-amplified tumors deserves further evaluation.
Trial registration number: NCT02034981.
Date of registration: 14 January 2014.
Conflict of interest statement
T. Aparicio has received honorarium from Roche, Ipsen, Amgen, Servier, Sanofi, and Bioven. L. Mineur has received honorarium from Merck, Servier, and Pierre Fabre and has received research grants from Amgen and Sanofi. R. Guimbaud has received honorarium from AAA, AstraZeneca, Amgen, BMS, Pierre Fabre, Novartis, Roche, and Servier. E. Samalin has received honorarium from Roche, Servier, Sanofi, MSD, BMS, Amgen, and Pierre Fabre. T. Lecomte has received honorarium from Ipsen, Amgen, Merck, Sanofi, Servier, Bayer, and Novartis. CA. Gomez-Roca has received honorarium from BMS, Roche, Pierre Fabre, Eisai, and Foundation Medicine, and has received research grants from Roche and BMS. G. Vassal has received honorarium from Bayer, Boehringer-Ingelheim, BMS, Celgene, Exelixis, Incyte Biosciences, Lilly, Merck, Novartis, Pfizer, Roche/Genentech, Servier and Takeda. N. Cozic, C. De La Fouchardière, E. Meriaux r, J. Plaza, F. Mary, PA. Haineaux, A. Gratet, J. Selves, Y. Menu, N. Colignon, L. Johnson, and F. Legrand declare that they have no conflicts of interest that might be relevant to the contents of this article.
Figures
References
-
- Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran S-E, Ilson DH, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(11):1467–1482. doi: 10.1016/S1470-2045(17)30566-1. - DOI - PMC - PubMed
-
- Shah MA, Bang Y-J, Lordick F, Alsina M, Chen M, Hack SP, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. 2017;3(5):620–627. doi: 10.1001/jamaoncol.2016.5580. - DOI - PMC - PubMed
-
- Malka D, François E, Penault-Llorca F, Castan F, Bouché O, Bennouna J, et al. FOLFOX alone or combined with rilotumumab or panitumumab as first-line treatment for patients with advanced gastroesophageal adenocarcinoma (PRODIGE 17-ACCORD 20-MEGA): a randomised, open-label, three-arm phase II trial. Eur J Cancer. 2019;115:97–106. doi: 10.1016/j.ejca.2019.04.020. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous