Verapamil as an Adjunct Therapy to Reduce tPA Toxicity in Hyperglycemic Stroke: Implication of TXNIP/NLRP3 Inflammasome
- PMID: 33847912
- PMCID: PMC8282727
- DOI: 10.1007/s12035-021-02384-z
Verapamil as an Adjunct Therapy to Reduce tPA Toxicity in Hyperglycemic Stroke: Implication of TXNIP/NLRP3 Inflammasome
Abstract
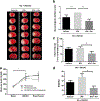
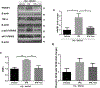
Thrombolytic therapy has remained quite challenging in hyperglycemic patients for its association with poor prognosis and increased hemorrhagic conversions. We recently showed that tissue plasminogen activator (tPA)-induced cerebrovascular damage is associated with thioredoxin-interacting protein (TXNIP) upregulation, which has an established role in the detrimental effects of hyperglycemia. In the present work, we investigated whether verapamil, an established TXNIP inhibitor, may provide protection against hyperglycemic stroke and tPA-induced blood-brain barrier (BBB) disruption. Acute hyperglycemia was induced by intraperitoneal administration of 20% glucose, 15 min prior to transient middle cerebral artery occlusion (tMCAO). Verapamil (0.15 mg/kg) or saline was intravenously infused with tPA at hyperglycemic reperfusion, 1 h post tMCAO. After 24 h of ischemia/reperfusion (I/R), mice were assessed for neurobehavioral deficits followed by sacrifice and evaluation of brain infarct volume, edema, and microbleeding. Alterations in TXNIP, inflammatory mediators, and BBB markers were further analyzed using immunoblotting or immunostaining techniques. As adjunctive therapy, verapamil significantly reduced tPA-induced BBB leakage, matrix metalloproteinase 9 (MMP-9) upregulation, and tight junction protein deregulation, which resulted in lesser hemorrhagic conversions. Importantly, verapamil strongly reversed tPA-induced TXNIP/NLRP3 (NOD-like receptor pyrin domain-containing-3) inflammasome activation and reduced infarct volume. This concurred with a remarkable decrease in high-mobility group box protein 1 (HMGB-1) and nuclear factor kappa B (NF-κB) stimulation, leading to less priming of NLRP3 inflammasome. This preclinical study supports verapamil as a safe adjuvant that may complement thrombolytic therapy by inhibiting TXNIP's detrimental role in hyperglycemic stroke.
Keywords: Acute hyperglycemia; Inflammasome; Stroke; Thioredoxin-interacting protein; Tissue plasminogen activator; Verapamil.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflicts of interests.
Figures






Similar articles
-
Tissue Plasminogen Activator Promotes TXNIP-NLRP3 Inflammasome Activation after Hyperglycemic Stroke in Mice.Mol Neurobiol. 2020 Jun;57(6):2495-2508. doi: 10.1007/s12035-020-01893-7. Epub 2020 Mar 14. Mol Neurobiol. 2020. PMID: 32172516 Free PMC article.
-
Acute Hyperglycemia Exacerbates Hemorrhagic Transformation after Embolic Stroke and Reperfusion with tPA: A Possible Role of TXNIP-NLRP3 Inflammasome.J Stroke Cerebrovasc Dis. 2022 Feb;31(2):106226. doi: 10.1016/j.jstrokecerebrovasdis.2021.106226. Epub 2021 Nov 27. J Stroke Cerebrovasc Dis. 2022. PMID: 34847489 Free PMC article.
-
Nod-like receptor protein 3 (NLRP3) inflammasome activation and podocyte injury via thioredoxin-interacting protein (TXNIP) during hyperhomocysteinemia.J Biol Chem. 2014 Sep 26;289(39):27159-27168. doi: 10.1074/jbc.M114.567537. Epub 2014 Aug 19. J Biol Chem. 2014. PMID: 25138219 Free PMC article.
-
The Role of NLRP3 Inflammasome in Cerebrovascular Diseases Pathology and Possible Therapeutic Targets.ASN Neuro. 2021 Jan-Dec;13:17590914211018100. doi: 10.1177/17590914211018100. ASN Neuro. 2021. PMID: 34053242 Free PMC article. Review.
-
Combination approaches to attenuate hemorrhagic transformation after tPA thrombolytic therapy in patients with poststroke hyperglycemia/diabetes.Adv Pharmacol. 2014;71:391-410. doi: 10.1016/bs.apha.2014.06.007. Epub 2014 Aug 22. Adv Pharmacol. 2014. PMID: 25307224 Review.
Cited by
-
Verapamil Attenuates the Severity of Tendinopathy by Mitigating Mitochondrial Dysfunction through the Activation of the Nrf2/HO-1 Pathway.Biomedicines. 2024 Apr 18;12(4):904. doi: 10.3390/biomedicines12040904. Biomedicines. 2024. PMID: 38672259 Free PMC article.
-
Intracerebroventricular calycosin attenuates cerebral ischemia-reperfusion injury in rats via HMGB1-dependent pyroptosis inhibition.Front Pharmacol. 2025 Jun 18;16:1596087. doi: 10.3389/fphar.2025.1596087. eCollection 2025. Front Pharmacol. 2025. PMID: 40606597 Free PMC article.
-
Thioredoxin interacting protein, a key molecular switch between oxidative stress and sterile inflammation in cellular response.World J Diabetes. 2021 Dec 15;12(12):1979-1999. doi: 10.4239/wjd.v12.i12.1979. World J Diabetes. 2021. PMID: 35047114 Free PMC article. Review.
-
The Potential of NLRP3 Inflammasome as a Therapeutic Target in Neurological Diseases.Mol Neurobiol. 2023 May;60(5):2520-2538. doi: 10.1007/s12035-023-03229-7. Epub 2023 Jan 21. Mol Neurobiol. 2023. PMID: 36680735 Review.
-
Endothelial Thioredoxin-Interacting Protein Depletion Reduces Hemorrhagic Transformation in Hyperglycemic Mice after Embolic Stroke and Thrombolytic Therapy.Pharmaceuticals (Basel). 2021 Sep 27;14(10):983. doi: 10.3390/ph14100983. Pharmaceuticals (Basel). 2021. PMID: 34681207 Free PMC article.
References
-
- Benjamin EJ, Muntner P, Bittencourt MS (2019) Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139:e56–e528 - PubMed
-
- McCormick MT, Muir KW, Gray CS, Walters MR (2008) Management of hyperglycemia in acute stroke: how, when, and for whom? Stroke 39:2177–2185 - PubMed
-
- Lindsberg PJ, Roine RO (2004) Hyperglycemia in acute stroke. Stroke 35:363–364 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

