Persistent peri-Heptacene: Synthesis and In Situ Characterization
- PMID: 33848044
- PMCID: PMC8251907
- DOI: 10.1002/anie.202102757
Persistent peri-Heptacene: Synthesis and In Situ Characterization
Abstract
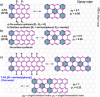
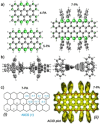
n-peri-Acenes (n-PAs) have gained interest as model systems of zigzag-edged graphene nanoribbons for potential applications in nanoelectronics and spintronics. However, the synthesis of n-PAs larger than peri-tetracene remains challenging because of their intrinsic open-shell character and high reactivity. Presented here is the synthesis of a hitherto unknown n-PA, that is, peri-heptacene (7-PA), in which the reactive zigzag edges are kinetically protected with eight 4-tBu-C6 H4 groups. The formation of 7-PA is validated by high-resolution mass spectrometry and in situ FT-Raman spectroscopy. 7-PA displays a narrow optical energy gap of 1.01 eV and exhibits persistent stability (t1/2 ≈25 min) under inert conditions. Moreover, electron-spin resonance measurements and theoretical studies reveal that 7-PA exhibits an open-shell feature and a significant tetraradical character. This strategy could be considered a modular approach for the construction of next-generation (3 N+1)-PAs (where N≥3).
Keywords: Scholl reaction; acenes; graphene; nanostructures; radicals.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- None
-
- Ruffieux P., Wang S., Yang B., Sánchez-Sánchez C., Liu J., Dienel T., Talirz L., Shinde P., Pignedoli C. A., Passerone D., Dumslaff T., Feng X., Müllen K., Fasel R., Nature 2016, 531, 489–492; - PubMed
-
- Wang X.-Y., Narita A., Müllen K., Nat. Rev. Chem. 2018, 2, 0100;
-
- None
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

