Highly Accurate Chip-Based Resequencing of SARS-CoV-2 Clinical Samples
- PMID: 33848173
- PMCID: PMC8056606
- DOI: 10.1021/acs.langmuir.0c02927
Highly Accurate Chip-Based Resequencing of SARS-CoV-2 Clinical Samples
Abstract
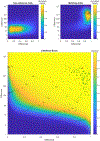
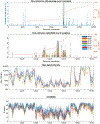
SARS-CoV-2 has infected over 128 million people worldwide, and until a vaccine is developed and widely disseminated, vigilant testing and contact tracing are the most effective ways to slow the spread of COVID-19. Typical clinical testing only confirms the presence or absence of the virus, but rather, a simple and rapid testing procedure that sequences the entire genome would be impactful and allow for tracing the spread of the virus and variants, as well as the appearance of new variants. However, traditional short read sequencing methods are time consuming and expensive. Herein, we describe a tiled genome array that we developed for rapid and inexpensive full viral genome resequencing, and we have applied our SARS-CoV-2-specific genome tiling array to rapidly and accurately resequence the viral genome from eight clinical samples. We have resequenced eight samples acquired from patients in Wyoming that tested positive for SARS-CoV-2. We were ultimately able to sequence over 95% of the genome of each sample with greater than 99.9% average accuracy.
Figures


References
-
- WHO, coronavirus disease (COVID-19) dashboard. Geneva: World Health Organization. Available online: https://covid19.who.int 2020.
-
- He X; Lau EHY; Wu P; Deng X; Wang J; Hao X; Lau YC; Wong JY; Guan Y; Tan X; Mo X; Chen Y; Liao B; Chen W; Hu F; Zhang Q; Zhong M; Wu Y; Zhao L; Zhang F; Cowling BJ; Li F; Leung GM, Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020, 26 (5), 672–675. - PubMed
-
- Drmanac R; Drmanac S; Baier J; Chui G; Coleman D; Diaz R; Gietzen D; Hou A; Jin H; Ukrainczyk T; Xu C, DNA Sequencing by Hybridization with Arrays of Samples or Probes. In DNA Arrays Methods and Protocols, Humana Press: Clifton, N.J., 2001; Vol. 170. - PubMed
-
- Shendure J; Mitra RD; Varma C; Church GM, Advanced sequencing technologies: methods and goals. Nat Rev Genet 2004, 5 (5), 335–44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

