Physiological effects of intraperitoneal versus subcutaneous insulin infusion in patients with diabetes mellitus type 1: A systematic review and meta-analysis
- PMID: 33848314
- PMCID: PMC8043377
- DOI: 10.1371/journal.pone.0249611
Physiological effects of intraperitoneal versus subcutaneous insulin infusion in patients with diabetes mellitus type 1: A systematic review and meta-analysis
Abstract
The intraperitoneal route of administration accounts for less than 1% of insulin treatment regimes in patients with diabetes mellitus type 1 (DM1). Despite being used for decades, a systematic review of various physiological effects of this route of insulin administration is lacking. Thus, the aim of this systematic review was to identify the physiological effects of continuous intraperitoneal insulin infusion (CIPII) compared to those of continuous subcutaneous insulin infusion (CSII) in patients with DM1. Four databases (EMBASE, PubMed, Scopus and CENTRAL) were searched beginning from the inception date of each database to 10th of July 2020, using search terms related to intraperitoneal and subcutaneous insulin administration. Only studies comparing CIPII treatment (≥ 1 month) with CSII treatment were included. Primary outcomes were long-term glycaemic control (after ≥ 3 months of CIPII inferred from glycated haemoglobin (HbA1c) levels) and short-term (≥ 1 day for each intervention) measurements of insulin dynamics in the systematic circulation. Secondary outcomes included all reported parameters other than the primary outcomes. The search identified a total of 2242 records; 39 reports from 32 studies met the eligibility criteria. This meta-analysis focused on the most relevant clinical end points; the mean difference (MD) in HbA1c levels during CIPII was significantly lower than during CSII (MD = -6.7 mmol/mol, [95% CI: -10.3 --3.1]; in percentage: MD = -0.61%, [95% CI: -0.94 -- 0.28], p = 0.0002), whereas fasting blood glucose levels were similar (MD = 0.20 mmol/L, [95% CI: -0.34-0.74], p = 0.47; in mg/dL: MD = 3.6 mg/dL, [95% CI: -6.1-13.3], p = 0.47). The frequencies of severe hypo- and hyper-glycaemia were reduced. The fasting insulin levels were significantly lower during CIPII than during CSII (MD = 16.70 pmol/L, [95% CI: -23.62 --9.77], p < 0.0001). Compared to CSII treatment, CIPII treatment improved overall glucose control and reduced fasting insulin levels in patients with DM1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
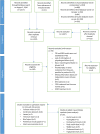

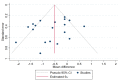



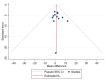
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

