Classification of neurological diseases using multi-dimensional CSF analysis
- PMID: 33848319
- PMCID: PMC8557345
- DOI: 10.1093/brain/awab147
Classification of neurological diseases using multi-dimensional CSF analysis
Abstract
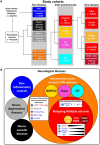
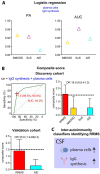
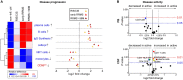
Although CSF analysis routinely enables the diagnosis of neurological diseases, it is mainly used for the gross distinction between infectious, autoimmune inflammatory, and degenerative disorders of the CNS. To investigate, whether a multi-dimensional cellular blood and CSF characterization can support the diagnosis of clinically similar neurological diseases, we analysed 546 patients with autoimmune neuroinflammatory, degenerative, or vascular conditions in a cross-sectional retrospective study. By combining feature selection with dimensionality reduction and machine learning approaches we identified pan-disease parameters that were altered across all autoimmune neuroinflammatory CNS diseases and differentiated them from other neurological conditions and inter-autoimmunity classifiers that subdifferentiate variants of CNS-directed autoimmunity. Pan-disease as well as diseases-specific changes formed a continuum, reflecting clinical disease evolution. A validation cohort of 231 independent patients confirmed that combining multiple parameters into composite scores can assist the classification of neurological patients. Overall, we showed that the integrated analysis of blood and CSF parameters improves the differential diagnosis of neurological diseases, thereby facilitating early treatment decisions.
Keywords: CNS autoimmunity; CSF; differential diagnosis; immune profile; multiple sclerosis.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Gruter T, Ott A, Meyer W, et al.Effects of IVIG treatment on autoantibody testing in neurological patients: Marked reduction in sensitivity but reliable specificity. J Neurol. 2020;267(3):715–720. - PubMed
-
- Ringelstein M, Harmel J, Zimmermann H, et al.; Neuromyelitis Optica Study Group (NEMOS). Longitudinal optic neuritis-unrelated visual evoked potential changes in NMO spectrum disorders. Neurology. 2020;94(4):e407–e418. - PubMed
-
- Dalmau J, Graus F.. Antibody-mediated encephalitis. N Engl J Med. 2018;378(9):840–851. - PubMed

