Therapeutic targeting of ATR yields durable regressions in small cell lung cancers with high replication stress
- PMID: 33848478
- PMCID: PMC8048383
- DOI: 10.1016/j.ccell.2021.02.014
Therapeutic targeting of ATR yields durable regressions in small cell lung cancers with high replication stress
Abstract
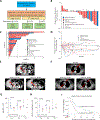
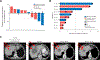
Small cell neuroendocrine cancers (SCNCs) are recalcitrant cancers arising from diverse primary sites that lack effective treatments. Using chemical genetic screens, we identified inhibition of ataxia telangiectasia and rad3 related (ATR), the primary activator of the replication stress response, and topoisomerase I (TOP1), nuclear enzyme that suppresses genomic instability, as synergistically cytotoxic in small cell lung cancer (SCLC). In a proof-of-concept study, we combined M6620 (berzosertib), first-in-class ATR inhibitor, and TOP1 inhibitor topotecan in patients with relapsed SCNCs. Objective response rate among patients with SCLC was 36% (9/25), achieving the primary efficacy endpoint. Durable tumor regressions were observed in patients with platinum-resistant SCNCs, typically fatal within weeks of recurrence. SCNCs with high neuroendocrine differentiation, characterized by enhanced replication stress, were more likely to respond. These findings highlight replication stress as a potentially transformative vulnerability of SCNCs, paving the way for rational patient selection in these cancers, now treated as a single disease.
Keywords: DNA damage response; DNA topoisomerases; SCLC; ataxia telangiectasia mutated and rad3 related; cell-cycle checkpoints; replication stress; small cell neuroendocrine cancers; translational research.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests H.D., A.Z., and F.T.Z. are employees of Merck KGaA, Darmstadt, Germany. B.E. is an employee of the EMD Serono Research & Development Institute Inc., Billerica, MA, USA; a business of Merck KGaA, Darmstadt, Germany. J.P. was an employee of the EMD Serono Research & Development Institute Inc., Billerica, MA, USA at the time of study. A.T., and Y.P. report research funding to the institution from the following entities: EMD Serono Research & Development Institute Inc., Billerica, MA, USA, AstraZeneca, Tarveda Therapeutics, and Prolynx Inc.
Figures



Comment in
-
A rational targeted therapy for platinum-resistant small-cell lung cancer.Cancer Cell. 2021 Apr 12;39(4):453-456. doi: 10.1016/j.ccell.2021.03.003. Cancer Cell. 2021. PMID: 33848475
References
-
- Anand J, Sun Y, Zhao Y, Nitiss KC, and Nitiss JL (2018). Detection of Topoisomerase Covalent Complexes in Eukaryotic Cells. Methods Mol Biol 1703, 283–299. - PubMed
-
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. (2005). DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864–870. - PubMed
-
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LVF, Kolettas E, Niforou K, Zoumpourlis VC, et al. (2006). Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

