Spatiotemporal regulation of hydrogen sulfide signaling in the kidney
- PMID: 33848877
- PMCID: PMC8065217
- DOI: 10.1016/j.redox.2021.101961
Spatiotemporal regulation of hydrogen sulfide signaling in the kidney
Abstract
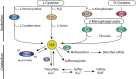
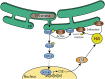
Hydrogen sulfide (H2S) has long been recognized as a putrid, toxic gas. However, as a result of intensive biochemical research in the past two decades, H2S is now considered to be the third gasotransmitter alongside nitric oxide (NO) and carbon monoxide (CO) in mammalian systems. H2S-producing enzymes are expressed in all organs, playing an important role in their physiology. In the kidney, H2S is a critical regulator of vascular and cellular function, although the mechanisms that affect (sub)cellular levels of H2S are not precisely understood. H2S modulates systemic and renal blood flow, glomerular filtration rate and the renin-angiotensin axis through direct inhibition of nitric oxide synthesis. Further, H2S affects cellular function by modulating protein activity via post-translational protein modification: a process termed persulfidation. Persulfidation modulates protein activity, protein localization and protein-protein interactions. Additionally, acute kidney injury (AKI) due to mitochondrial dysfunction, which occurs during hypoxia or ischemia-reperfusion (IR), is attenuated by H2S. H2S enhances ATP production, prevents damage due to free radicals and regulates endoplasmic reticulum stress during IR. In this review, we discuss current insights in the (sub)cellular regulation of H2S anabolism, retention and catabolism, with relevance to spatiotemporal regulation of renal H2S levels. Together, H2S is a versatile gasotransmitter with pleiotropic effects on renal function and offers protection against AKI. Unraveling the mechanisms that modulate (sub)cellular signaling of H2S not only expands fundamental insight in the regulation of functional effects mediated by H2S, but can also provide novel therapeutic targets to prevent kidney injury due to hypoxic or ischemic injury.
Keywords: Gasotransmitter; Hydrogen sulfide; Hypoxia; Ischemia-reperfusion injury; Kidney; Persulfidation.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Duffus J. H. International. Encyclopedia of Toxicology. third ed. 2014. Union of pure and applied chemistry. - DOI
-
- Administration O.S., Safety H. Health topics: hydrogen sulfide. Occupation, retrieved from. 1970. https://www.osha.gov/SLTC/hydrogen
-
- Tang S., Huang D., An N., Chen D., Zhao D. A novel pathway for the production of H2S by DAO in rat jejunum. Neuro Gastroenterol. Motil. 2016;28:687–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

