Innate Immune Cells in the Adipose Tissue in Health and Metabolic Disease
- PMID: 33849008
- PMCID: PMC8787575
- DOI: 10.1159/000515117
Innate Immune Cells in the Adipose Tissue in Health and Metabolic Disease
Abstract
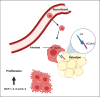
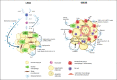
Metabolic disorders, such as obesity, type 2 diabetes mellitus, and nonalcoholic fatty liver disease, are characterized by chronic low-grade tissue and systemic inflammation. During obesity, the adipose tissue undergoes immunometabolic and functional transformation. Adipose tissue inflammation is driven by innate and adaptive immune cells and instigates insulin resistance. Here, we discuss the role of innate immune cells, that is, macrophages, neutrophils, eosinophils, natural killer cells, innate lymphoid type 2 cells, dendritic cells, and mast cells, in the adipose tissue in the healthy (lean) and diseased (obese) state and describe how their function is shaped by the obesogenic microenvironment, and humoral, paracrine, and cellular interactions. Moreover, we particularly outline the role of hypoxia as a central regulator in adipose tissue inflammation. Finally, we discuss the long-lasting effects of adipose tissue inflammation and its potential reversibility through drugs, caloric restriction, or exercise training.
Keywords: Adipose tissue; Chronic inflammation; Endothelium; Hypoxia; Innate immune cells; Obesity; Physical exercise.
© 2021 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
The role of innate immune cells in obese adipose tissue inflammation and development of insulin resistance.Thromb Haemost. 2013 Mar;109(3):399-406. doi: 10.1160/TH12-09-0703. Epub 2013 Jan 31. Thromb Haemost. 2013. PMID: 23364297 Review.
-
Regulation of metabolism by the innate immune system.Nat Rev Endocrinol. 2016 Jan;12(1):15-28. doi: 10.1038/nrendo.2015.189. Epub 2015 Nov 10. Nat Rev Endocrinol. 2016. PMID: 26553134 Review.
-
Innate immune cells in the adipose tissue.Rev Endocr Metab Disord. 2018 Dec;19(4):283-292. doi: 10.1007/s11154-018-9451-6. Rev Endocr Metab Disord. 2018. PMID: 29922964 Review.
-
Role of Innate lymphoid Cells in Obesity and Insulin Resistance.Front Endocrinol (Lausanne). 2022 Apr 27;13:855197. doi: 10.3389/fendo.2022.855197. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35574038 Free PMC article. Review.
-
From neutrophils to macrophages: differences in regional adipose tissue depots.Obes Rev. 2016 Jan;17(1):1-17. doi: 10.1111/obr.12335. Epub 2015 Dec 14. Obes Rev. 2016. PMID: 26667065 Review.
Cited by
-
LIGHT/TNFSF14 Affects Adipose Tissue Phenotype.Int J Mol Sci. 2024 Jan 5;25(2):716. doi: 10.3390/ijms25020716. Int J Mol Sci. 2024. PMID: 38255789 Free PMC article.
-
Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs.Int J Mol Sci. 2024 Jun 27;25(13):7076. doi: 10.3390/ijms25137076. Int J Mol Sci. 2024. PMID: 39000187 Free PMC article. Review.
-
Cytokine signatures in post-endoscopic retrograde cholangiopancreatography pancreatitis: a pilot study.Ann Gastroenterol. 2024 Nov-Dec;37(6):734-741. doi: 10.20524/aog.2024.0922. Epub 2024 Oct 23. Ann Gastroenterol. 2024. PMID: 39568712 Free PMC article.
-
Interleukin-33 and Obesity-Related Inflammation and Cancer.Encyclopedia (Basel, 2021). 2024 Dec;4(4):1770-1789. doi: 10.3390/encyclopedia4040117. Epub 2024 Nov 23. Encyclopedia (Basel, 2021). 2024. PMID: 40236667 Free PMC article.
-
Adipocyte deletion of the oxygen-sensor PHD2 sustains elevated energy expenditure at thermoneutrality.Nat Commun. 2024 Aug 29;15(1):7483. doi: 10.1038/s41467-024-51718-7. Nat Commun. 2024. PMID: 39209825 Free PMC article.
References
-
- Chmelar J, Chung KJ, Chavakis T. The role of innate immune cells in obese adipose tissue inflammation and development of insulin resistance. Thromb Haemost. 2013;109((3)):399–406. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

