Plasma membrane integrity: implications for health and disease
- PMID: 33849525
- PMCID: PMC8042475
- DOI: 10.1186/s12915-021-00972-y
Plasma membrane integrity: implications for health and disease
Abstract
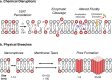
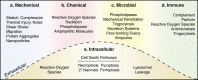
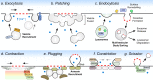
Plasma membrane integrity is essential for cellular homeostasis. In vivo, cells experience plasma membrane damage from a multitude of stressors in the extra- and intra-cellular environment. To avoid lethal consequences, cells are equipped with repair pathways to restore membrane integrity. Here, we assess plasma membrane damage and repair from a whole-body perspective. We highlight the role of tissue-specific stressors in health and disease and examine membrane repair pathways across diverse cell types. Furthermore, we outline the impact of genetic and environmental factors on plasma membrane integrity and how these contribute to disease pathogenesis in different tissues.
Keywords: Cell biology; Disease; Lipid peroxidation; Membrane damage; Membrane repair; Plasma membrane; Pore formation; Tissue injury; Vesicle trafficking.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- McNeil PL, Ito S. Molecular traffic through plasma membrane disruptions of cells in vivo. J Cell Sci. 1990;96:549–556. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

