Circ_0116061 regulated the proliferation, apoptosis, and inflammation of osteoarthritis chondrocytes through regulating the miR-200b-3p/SMURF2 axis
- PMID: 33849596
- PMCID: PMC8045261
- DOI: 10.1186/s13018-021-02391-9
Circ_0116061 regulated the proliferation, apoptosis, and inflammation of osteoarthritis chondrocytes through regulating the miR-200b-3p/SMURF2 axis
Abstract
Background: Circular RNA (circRNA) has been shown to be associated with osteoarthritis (OA) progression. Circ_0116061 has been found to be highly expressed in OA cartilage tissues, but its role and mechanism in OA progression remain unclear.
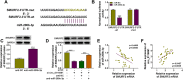
Methods: Expression levels of circ_0116061, microRNA (miR)-200b-5p, and Smad ubiquitin regulatory factor 2 (SMURF2) were detected using quantitative real-time PCR. The proliferation and apoptosis of cells were measured using cell counting kit 8 (CCK8) assay, colony formation assay, and flow cytometry. Furthermore, the protein levels of proliferation-related marker, apoptosis-related markers, inflammatory factors, and SMURF2 were tested using western blot (WB) analysis. In addition, the interaction between miR-200b-3p and circ_0116061 or SMURF2 was examined using dual-luciferase reporter assay and biotin-labeled RNA pull-down assay.
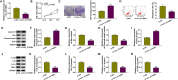
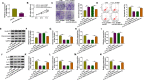
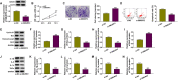
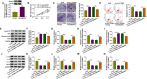
Results: Circ_0116061 and SMURF2 were highly expressed, and miR-200b-3p was lowly expressed in OA cartilage tissues. Knockdown of circ_0116061 could promote the proliferation and inhibit the apoptosis and inflammation of OA chondrocytes. MiR-200b-3p could be sponged by circ_0116061, and its inhibitor could reverse the regulation of circ_0116061 silencing on the biological functions of OA chondrocytes. SMURF2 was a target of miR-200b-3p, and its expression was positively regulated by circ_0116061. Silencing of SMURF2 also could enhance the proliferation and suppress the apoptosis and inflammation of OA chondrocytes. Furthermore, the regulation of circ_0116061 silencing on the biological functions of OA chondrocytes also could be reversed by SMURF2 overexpression.
Conclusion: Our data showed that circ_0116061 might regulate the miR-200b-3p/SMURF2 axis to promote the progression of OA.
Keywords: Chondrocytes; Circ_0116061; MiR-200b-3p; Osteoarthritis; SMURF2.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
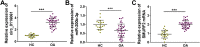

References
-
- Belluzzi E, Macchi V, Fontanella CG, Carniel EL, Olivotto E, Filardo G, Sarasin G, Porzionato A, Granzotto M, Pozzuoli A, et al. Infrapatellar fat pad gene expression and protein production in patients with and without osteoarthritis. Int J Mol Sci. 2020;21(17):6016. doi: 10.3390/ijms21176016. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

